BLENREP dosing regimen
Not a healthcare professional? Visit our Public site
Not a healthcare professional? Visit our Public site

has been added to your basket
59
For Great Britain (GB) healthcare professionals only - BLENREP is not available in Northern Ireland (NI).
BLENREP has a conditional marketing authorisation.
BLENREP offers a BCMA-targeted therapy to your 5L+ patients with triple- class refractory myeloma, as a monotherapy in the outpatient setting*1
BLENREP is only available via the private market.
BLENREP must be reconstituted and diluted by a healthcare professional prior to administration as an intravenous infusion.1
The recommended dose of BLENREP is:1
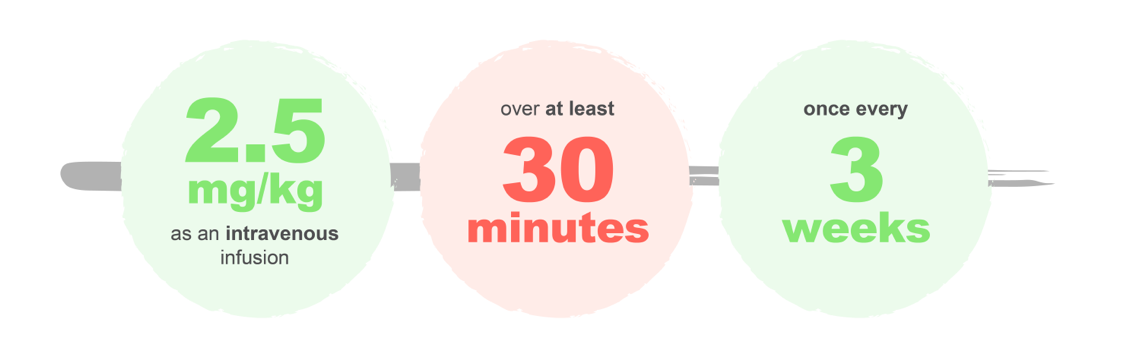
Treatment should be continued until disease progression or unacceptable toxicity.1
Learn more about how to manage side effects with dose modifications, so you can aim to keep your patients on treatment.
BLENREP is indicated as monotherapy for the treatment of multiple myeloma in adult patients, who have received at least four prior therapies and whose disease is refractory to at least one proteasome inhibitor, one immunomodulatory agent, and an anti-CD38 monoclonal antibody, and who have demonstrated disease progression on the last therapy.1
Prescribing information can be found at the top of this webpage, or here.
Abbreviations
AE, adverse event; BCMA, B cell maturation antigen; BCVA, best corrected visual acuity; SmPC, Summary of Product Characteristics.
References
▼This medicine is subject to additional monitoring. This will allow quick identification of new safety information.
Adverse events should be reported. Reporting forms and information can be found at https://yellowcard.mhra.gov.uk/ or search for MHRA Yellowcard in the Google Play or Apple App store. Adverse events should also be reported to GlaxoSmithKline on 0800 221 441 or email us on UKSafety@gsk.com.
© 2022 GSK Group of Companies or its licensor. Trademarks are the property of their respective owners.
February 2024 | PM-GB-BLM-WCNT-220006 (V5.0)
