DREAMM-2 safety summary
Not a healthcare professional? Visit our Public site
Not a healthcare professional? Visit our Public site

has been added to your basket
59
For Great Britain (GB) healthcare professionals only. Not available in Northern Ireland (NI).
BLENREP has a conditional marketing authorisation.
BLENREP has a generally well tolerated and manageable safety profile1
BLENREP is only available via the private market.
In the final analysis (40 months follow-up) of the DREAMM-2 trial, BLENREP (administered as 2.5 mg/kg every 3 weeks) delivered an overall response rate of 32% (n=31/97; 95% CI 21.7-43.6) in triple-refractory patients with multiple myeloma. 58% (n=18) of responding patients achieved a ≥VGPR. Responses to BLENREP were fast, deep and durable.2

of affected patients (n=49/67) had recovered from their first keratopathy event by the final analysis data cut off2‡

of responses (n=14/16) were sustained or deepened, despite prolonged dose delays (from post-hoc analysis in 13 month follow up).3
†Discontinuation included three patient with keratopathy, one patient with blurred vision, and one patient with reduced visual acuity.2
‡Recovery is defined as resolution or return to baseline. DREAMM-2 13-month follow up.
| BLENREP, 2.5 mg/kg (n=95) |
|||
|---|---|---|---|
| Keratopathy |
Blurred vision | BCVA reduced to 20/50 or worse | |
| Incidence, % (n) | 71% (67) | 25% (24) | 19% (18) |
| Median time to resolution of first event, days (range) | 120.0* (8–858) | 43.0 (6–895) | 21.5 † (7-64) |
| Recovered as of last assessment, % (n) | 73% (49/67) | 79% (19/24) | 89% (44/46) |
Adapted from Ramasamy, Ket al. BSH 2023
*Duration defined as time from onset of any keratopathy event to first time subject is free from event.
†Resolution defined as having a post-baseline score ≥20/50 or no equivalent value in either eye.
A gap of ≥1 day was required between resolution of first and occurrence of second event.
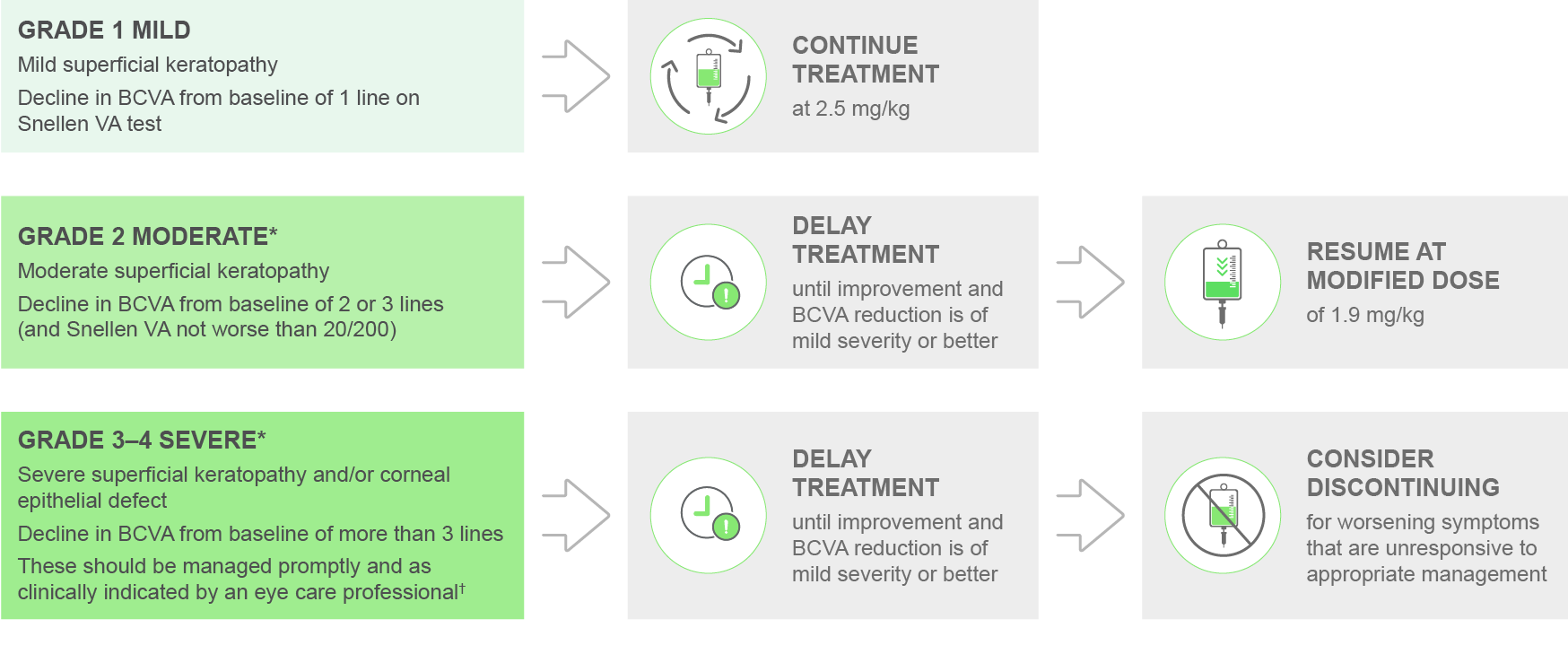
Adapted from BLENREP SmPC. Please see the SmPC for guidance on dosing and dose delays, and for further information.
BVCA, best-corrected visual acuity.
*The severity category is defined by the most severely affected eye as both eyes may not be affected to the same degree.1
†An eye care professional can be an ophthalmologist or optometrist.
Mild superficial keratopathy is documented worsening from baseline, with or without symptoms.1
Moderate superficial keratopathy is with or without patchy microcyst-like deposits, sub-epithelial haze (peripheral), or a new peripheral stromal opacity.1
Severe superficial keratopathy is with or without diffuse microcyst-like deposits involving the central cornea, sub-epithelial haze (central), or a new central stromal opacity.1
A corneal defect may lead to corneal ulcers. These should be managed promptly and as clinically indicated by an eye care professional.1
Clinical outcomes in a post-hoc analysis of patients who had prolonged dose delays (in which ≥3 treatment cycles were missed) in the 13-month follow up, after initially responding to BLENREP 2.5mg/kg (n=16/31)*3

Adapted from Lonial S, et al. Cancer. 2021.
*Percentages do not add up to 100% due to rounding. Rounded down due to small numbers.
†Indicates patients with elevated paraproteins reported during the delays, though these elevated paraproteins did not meet progressive disease criteria.3
One patient developed progressive disease 6 weeks into delay and one patient developed progressive disease 3 weeks after delay.3
With BLENREP, there was no change in overall patient-reported Global Health Status/QoL, Physical Functioning, or Role Functioning domain scores of the EORTC-QLQ-C30 at the final readout vs. baseline. This included patients with a minimal meaningful withinpatient reduction in vision-related function by Ocular Surface Disease Index (OSDI).3
BL, baseline; EORTC-QLQ-C30, European Organisation for Research and Treatment of cancer quality of life questionnaire; OSDI, Ocular Surface Disease Index; QoL, quality of life.
The European Organisation for Research and Treatment of Cancer quality of life questionnaire (EORTC-QLQ-C30) is a 30 item, cancerspecific measure of global health-related quality of life. The Ocular Surface Disease Index (OSDI) is an ophthalmic vision–related PRO questionnaire used to characterise the impact of corneal events on patient symptoms and visual function. In DREAMM-2, patients completed PRO questionnaires electronically at baseline and Q3W during treatment.
BLENREP is indicated as monotherapy for the treatment of multiple myeloma in adult patients, who have received at least four prior therapies and whose disease is refractory to at least one proteasome inhibitor, one immunomodulatory agent, and an anti-CD38 monoclonal antibody, and who have demonstrated disease progression on the last therapy.1
Prescribing information can be found at the top of this webpage, or here.
Abbreviations
AE, adverse event; BCVA, best corrected visual acuity; BL, baseline; CI, confidence interval; DREAMM-2, DRiving Excellence in Approaches to Multiple Myeloma 2; EORTC-QLQ-C30, European Organisation for Research and Treatment of cancer quality of life questionnaire; GB, Great Britain; NI, Northern Ireland ORR, Overall Response Rate; OSDI, Ocular Surface Disease Index; PS, performance status; Q3W, every 3 weeks; QoL, quality of life; R-ISS, Revised International Staging System; SAE, Severe adverse event; SmPC, Summary of Product Characteristics; VGPR, Very good partial response;
References
▼This medicine is subject to additional monitoring. This will allow quick identification of new safety information.
Adverse events should be reported. Reporting forms and information can be found at https://yellowcard.mhra.gov.uk/ or search for MHRA Yellowcard in the Google Play or Apple App store. Adverse events should also be reported to GlaxoSmithKline on 0800 221 441 or email us on UKSafety@gsk.com.
© 2022 GSK Group of Companies or its licensor. Trademarks are the property of their respective owners.
February 2024 | PM-GB-BLM-WCNT-220004 (V5.0)
