This is a fictional patient for illustrative purposes.
Anoro Ellipta is indicated as a once-daily maintenance bronchodilator treatment to relieve symptoms in adult patients with COPD.
Did you know, five out of ten COPD patients reported significant breathlessness (mMRC≥2) despite maintenance therapy with a long-acting mono-bronchodilator*2

*This single-visit study conducted in 56 primary and speciality care centres in the USA investigated symptom burden, QoL and comorbidity in COPD patients receiving maintenance therapy with long-acting bronchodilator (LABD) monotherapy. The study found that 64% (n=689) of patients enrolled had spirometry consistent with a diagnosis of COPD and of these, 77%, 15% and 8% were using tiotropium, formoterol and salmeterol, respectively. Regardless of the severity of lung function impairment, the study found that approximately 50% of patients on LABD monotherapy had mMRC scores ≥2.2
Are any of your patients on maintenance monotherapy still suffering from breathlessness?
Or still experiencing symptoms?
How does Anoro Ellipta (a LAMA/LABA) compare with tiotropium HandiHaler (a LAMA) for improvement in trough FEV1?
Change in trough FEV1 at day 169
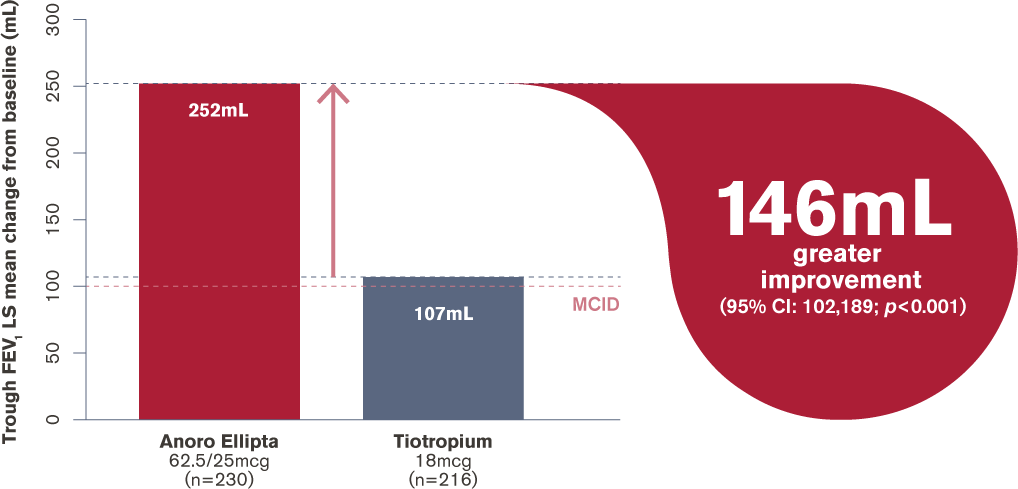

LS, least squares; RCT, randomised controlled trial;
MCID (minimum clinically important difference) = 100mL
Adapted from Maleki-Yazdi et al. 20163
What could an improvement in lung function mean for your COPD patients?

A post hoc, pooled analysis of 23 RCTs of long-acting bronchodilators* in COPD (n=23, 213) found that improvements in trough FEV1 post bronchodilator therapy were associated with improvements in patient-reported outcomes (PROs) such as St George's Respiratory Questionnaire (SGRQ) and Transition Dyspnoea Index (TDI)1
*LAMA, LABA and the LAMA/LABA Ultibro.1
How does Anoro Ellipta (a LAMA/LABA) compare with tiotropium HandiHaler (a LAMA) for reduction in rescue medicine use?
Change in rescue medication use (an additional efficacy endpoint) in three 24-week RCTs (in which the primary endpoint was trough FEV1)*
Maleki-Yazdi et al. 20145
Decramer et al. 20146
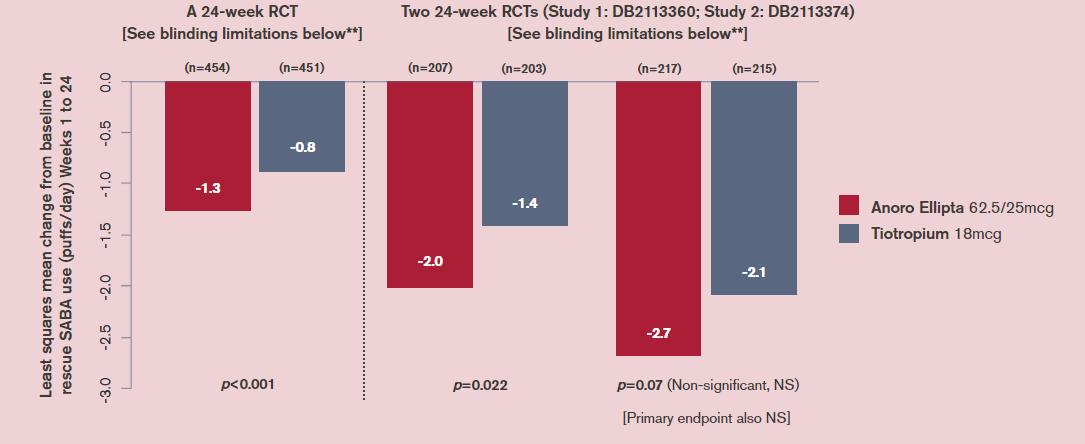

LS, least squares; NS, not statistically significant.
*LABA, tiotropium, and ICS/LABA therapies were stopped ≥2 days, ≥14 days and ≥30 days before screening visit, respectively.
**Whilst patients and physicians were masked to assigned treatment, tiotropium capsules (also, blister packs and inhalers) had markings not present on the placebo capsules. Labels were used to cover identifying information/markings on blister packs/inhalers and all studies had a parallel-group design so capsule type, blister packs and inhalers were consistent for patients for the study duration. Staff involved with efficacy and safety assessments were also absent from dosing in the clinic to guard against the possibility of them identifying which capsules the patient was receiving and making correct inferences.
Get started with Anoro Ellipta
References
- Donohue JF et al. Pulm Pharmacol Ther 2018;49:11–19.
- Dransfield MT, et al. Prim Care Respir J 2011; 20:46–53
- Maleki-Yazdi M et al. Adv Ther 2016; 33:2188–2199
- GSK data on file RF/UCV/0112/15
- Maleki-Yazdi MR et al. Respir Med 2014;108:1752–1760
- Decramer M et al. Lancet Respir Med 2014;2:472–486.
Adverse events should be reported directly to the Health Products Regulatory Authority (HPRA) on their website: www.hpra.ie.
Adverse events should also be reported to GlaxoSmithKline on 1800 244 255.
▼This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions.
Anoro is a registered trademark of the GlaxoSmithKline group of companies.

PM-IE-UCV-WCNT-210012 January 2024







