Help reduce the impact that COPD exacerbations have on your patients1-5
Even a single exacerbation can lead to poorer outcomes1-3
TRELEGY Ellipta: statistically superior reduction in annual rate of moderate/severe exacerbations vs. budesonide/formoterol4
TRELEGY Ellipta is the only Triple Therapy to provide statistically superior reduction in hospitalised exacerbations vs. a LAMA/LABA (UMEC/VI)5
Statistically superior reduction in annual rate of moderate/severe exacerbations vs. budesonide/formoterol4
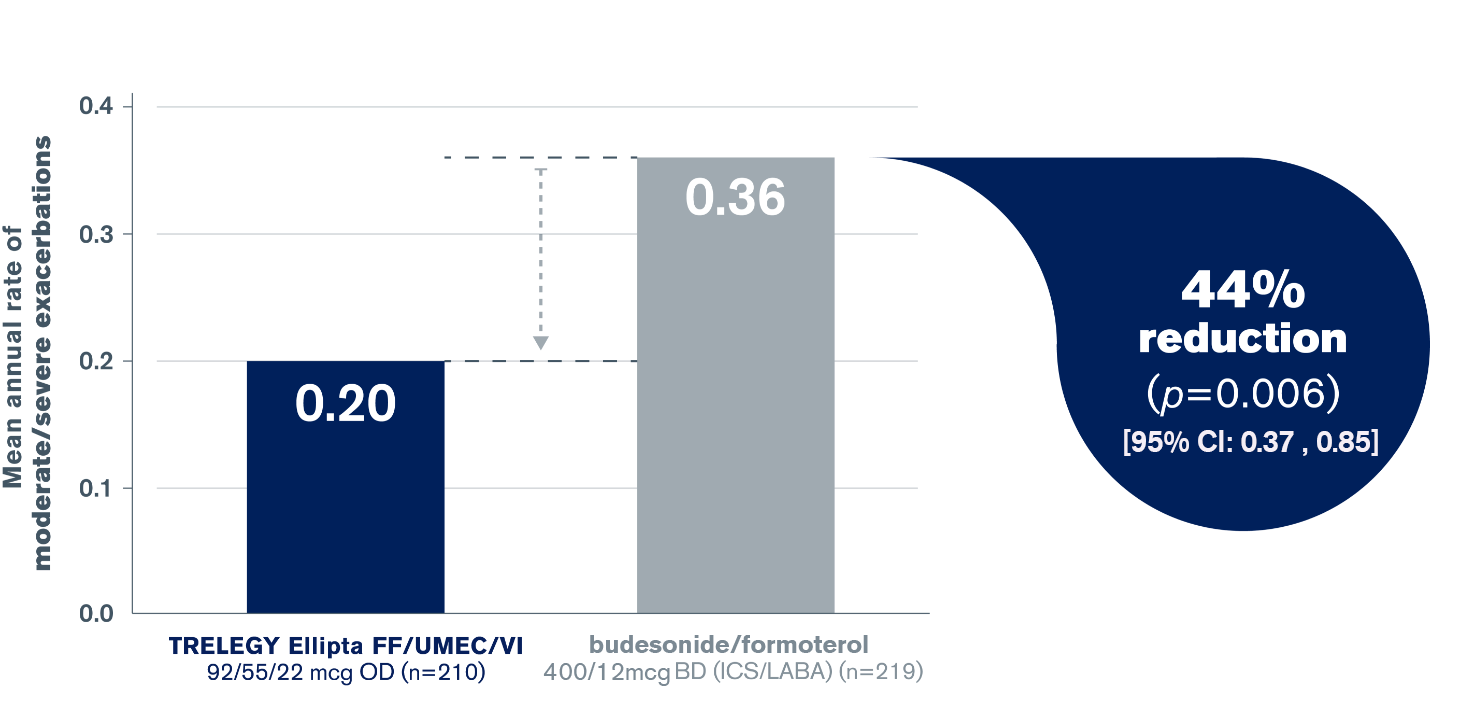
Adapted from Lipson et al. 20174
FULFIL was a Phase III, randomised, double-blind, double-dummy, parallel-group, multicentre, 24-week study designed to assess the efficacy and safety of the single-inhaler Triple Therapy TRELEGY Ellipta OD (FF/UMEC/VI) vs. BUD/FOR, with an extension to 52 weeks in a subset of patients4
The extension population was comprised of a subset of patients in the ITT population (N=1,810) who were enrolled into the 52-week extension phase of the study and remained on blinded treatment for up to 52 weeks4
The co-primary endpoints of change from baseline in trough FEV1 and SGRQ score at Week 24 were both met in this study4
Statistically superior reduction in annual rate of hospitalised exacerbations vs. a LAMA/LABA (UMEC/VI)5
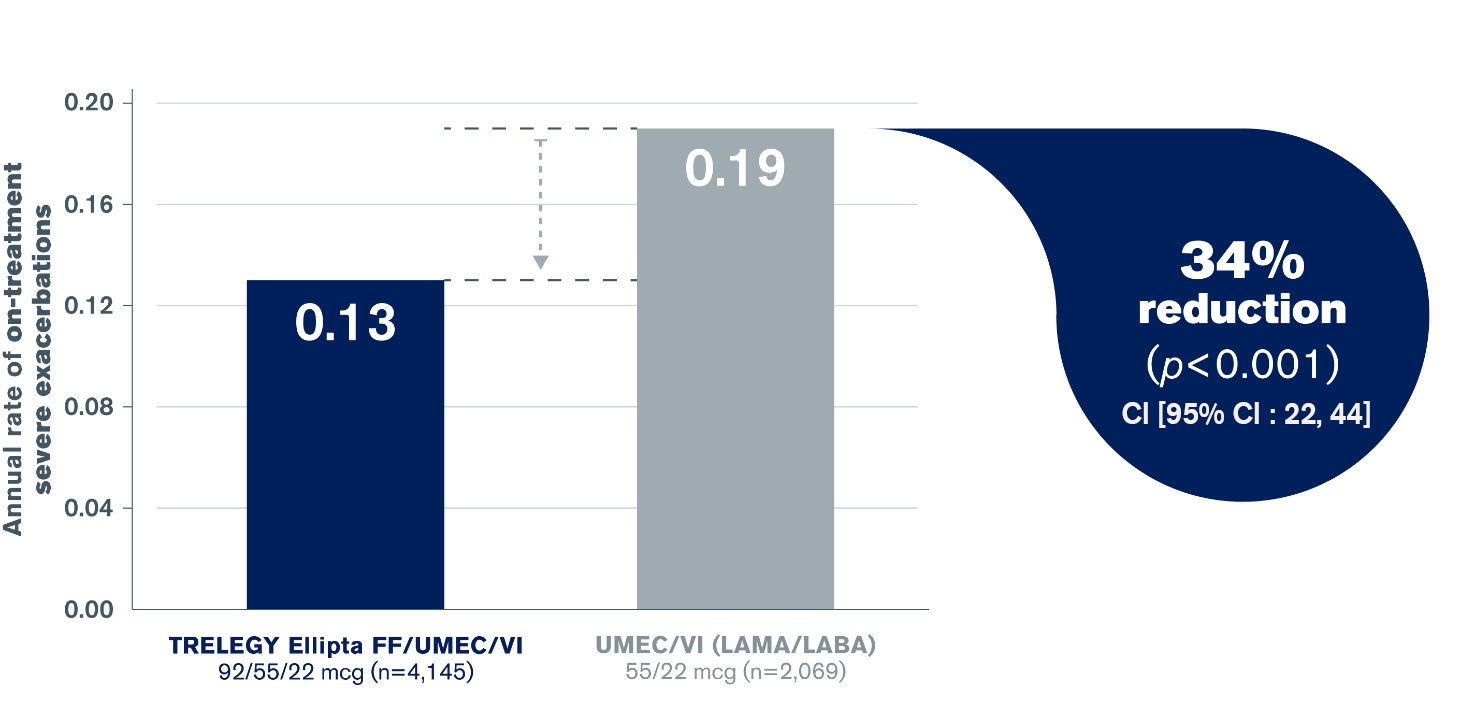
Adapted from Lipson et al. 20185
Co-primary endpoints of annual rate of on-treatment moderate/severe exacerbations for TRELEGY Ellipta vs. both FF/VI and UMEC/VI were met
Key trial design
Get started with Trelegy Ellipta
Footnotes
BD, twice daily; BUD, budesonide; CAT, COPD assessment test; FEV1, forced expiratory volume in one second; FF, fluticasone furoate; FOR, formoterol; ICS, inhaled corticosteroid; ITT, intent-to-treat; LABA, long-acting ß2-agonist; LAMA, long-acting muscarinic antagonist; OD, once daily; QoL, quality of life; SGRQ, St George’s Respiratory Questionnaire; UMEC, umeclidinium; VI, vilanterol
TRELEGY Ellipta OD is indicated for maintenance treatment in adult patients with moderate-to-severe chronic obstructive pulmonary disease (COPD) who are not adequately treated by a combination of an ICS and a LABA, or a combination of a LAMA and a LABA6
References
- Celli B et al. Am J Respir Crit Care Med 2008; 178:332–338.
- Donaldson GC et al. Chest 2010; 137:1091–1097.
- Soler-Cataluna JJ et al. Thorax 2005; 60:925–931.
- Lipson DA et al. Am J Crit Care Med 2017; 196:438–446.
- Lipson DA et al. N Engl J Med 2018; 378:1671–1680.
- TRELEGY Ellipta SmPC, 2022. Available at www.medicines.ie. Accessed December 2022.
Adverse events should be reported directly to the Health Products Regulatory Authority (HPRA) on their website: www.hpra.ie . Adverse events should also be reported to GlaxoSmithKline on 1800 244 255.
Trelegy is a registered trademark of the GlaxoSmithKline group of companies.
TRELEGY Ellipta was developed in collaboration with

Last Updated: December 2022 PM-IE-FVU-WCNT-220022








