4
Reduce the day-to-day burden of severe refractory eosinophilic asthma in your patients
The MUSCA trial adds to the increasing body of clinical evidence demonstrating the benefit of Nucala for patients with severe refractory eosinophilic asthma. The design of MUSCA adds further confidence to the evidence that add-on treatment with Nucala can bring significant and clinically meaningful changes to patients’ everyday lives through improvements in health-related quality of life (HRQoL), lung function and measures of disease control, compared with standard of care.
Early, sustained and clinically meaningful improvements in HRQoL vs. standard of care
The personal burden of severe asthma can be high, and its impact on patients’ quality of life is known to increase with disease severity. 1-3 And for patients who experience exacerbations, HRQoL is significantly worse than for those who do not, with further detriment to those who are hospitalised as a result of exacerbations. 4 Hence, there is a real need for treatments that not only demonstrate safety and efficacy in objective measures, such as exacerbation reduction and lung function improvement, but also provide benefits that patients can feel in their everyday lives.
MUSCA characterised the impact of Nucala treatment on patient HRQoL
Although outcomes relating to HRQoL are often assessed in clinical trials, this is generally as a secondary outcome of interest, and so the trial may not be appropriately powered to fully characterise these changes. MUSCA was a Phase IIIb, randomised, double-blind, placebo-controlled, 24-week trial specifically designed to assess the impact of add-on treatment with Nucala on HRQoL (assessed with the St. George’s Respiratory Questionnaire; SGRQ), compared with standard of care, in patients with severe refractory eosinophilic asthma. 5
Secondary and other endpoints assessed in the trial included disease control (assessed with the Asthma Control Questionnaire-5; ACQ-5), lung function (pre-bronchodilator forced expiratory volume in 1 second; FEV1) and annualised rate of clinically significant exacerbations.* Safety outcomes were also evaluated. 5
More information about the study design of MUSCA
Significant improvements in HRQoL vs. placebo from as early as Week 4
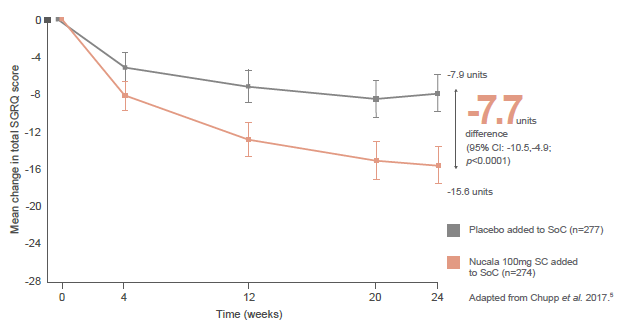
In the primary endpoint, patients receiving Nucala experienced a statistically significant improvement in HRQoL at Week 24 compared with placebo, with a difference in total SGRQ score that was almost twice that which is considered to be the minimal clinically important difference (-7.7 unit difference; a difference of 4.0 units is considered clinically meaningful). 5 6 Significant differences compared with placebo were evident from as early as Week 4. 5 7
Improvement in SGRQ total score from baseline vs. placebo (primary endpoint) 5
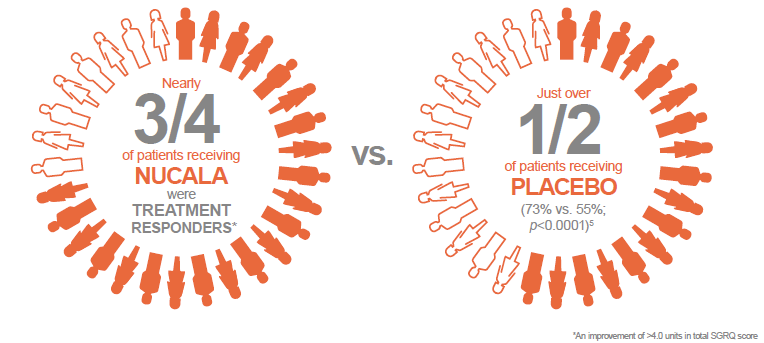
Moreover, at this time, nearly three quarters of patients receiving Nucala were treatment responders, in terms of HRQoL (an improvement of at least 4.0 units in total SGRQ score), compared with just over half of patients receiving placebo (73% vs. 55%; p<0.0001). 5
Greater improvements in lung function and superior asthma control vs. placebo from Week 4
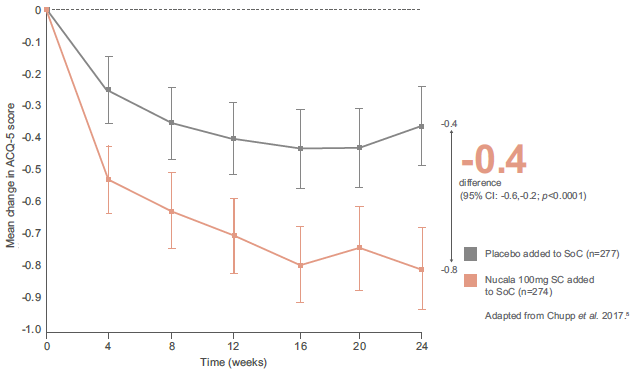
In secondary outcomes, Nucala demonstrated a consistently superior efficacy profile compared with placebo, with early and sustained improvements in lung function and asthma control. For both outcomes, differences were observed from the first assessment (Week 4), with a clear separation between groups maintained throughout the study period. 5
Improvement in lung function from baseline (pre-bronchodilator FEV1) vs. placebo (secondary endpoint) 5

Improvement in asthma control from baseline (ACQ-5) vs. placebo (secondary endpoint) 5
MUSCA reinforces Nucala’s proven exacerbation reduction profile
Patients with poor symptom control are at risk of exacerbations. 8 In turn, exacerbations have been shown to be associated with significantly reduced HRQoL compared with patients who do not exacerbate. 4 Exacerbations are not only burdensome to patients, but also significantly increase the cost of healthcare, with increasing costs for severe exacerbations – one study found that severe asthma exacerbations cost 4x more than mild, intermittent asthma exacerbations. 9 These data highlight the need to develop management strategies and treatments in severe asthma that reduce the frequency of exacerbations.
The Phase III MENSA study demonstrated a 53% reduction in clinically significant exacerbations* in patients receiving add-on treatment with Nucala for 32 weeks compared with placebo (0.83/year vs. 1.74/year; p<0.001). 10 This effect was seen to be durable in the open-label extension study, COSMOS, with sustained exacerbation rates over a further 52 weeks of treatment. 11
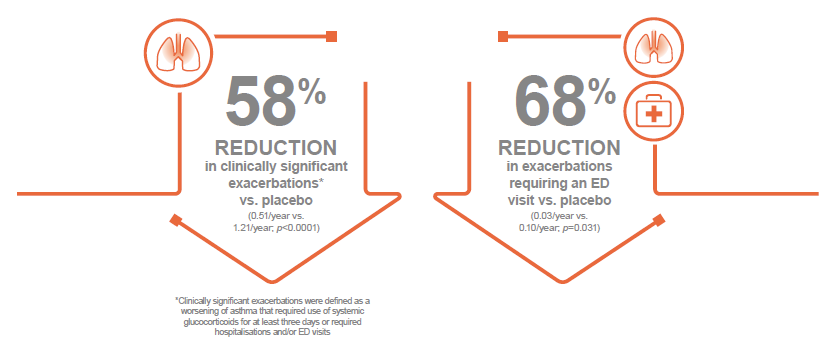
In the MUSCA trial, clinically significant exacerbations* were statistically significantly reduced by 58% at Week 24 in patients receiving Nucala compared with those receiving placebo (0.51/year vs. 1.21/year; p<0.0001). Furthermore, there was a 68% reduction in exacerbations requiring an emergency department (ED) visit or hospital admission compared with placebo (0.03/year vs. 0.10/year; p=0.031). 5
These data further demonstrate the clinical value that Nucala can bring for patients with severe refractory eosinophilic asthma.
Nucala had a similar incidence of adverse events vs. placebo
In addition to the demonstration of a consistent efficacy profile, MUSCA showed that Nucala was generally well tolerated, with a safety profile that was consistent with previous clinical trials. These findings confirm the positive safety profile of Nucala for use in patients with severe refractory eosinophilic asthma. 5
More information about the efficacy and safety data from MUSCA
*Clinically significant exacerbations were defined as a worsening of asthma that required use of systemic glucocorticoids for at least three days or required hospitalisations and/or ED visits 10
References
- Aburuz S et al. Respirology 2007; 12:227–233.
- Almada Lobo F, Almada-Lobo B. J Asthma 2008; 45:27–32.
- Wakefield S et al. Am J Respir Crit Care Med 2011; 183:A5467.
- Lloyd A et al. Prim Care Respir J 2007; 16:22–27.
- Chupp GL et al. Lancet Respir Med 2017; 5:390–400.
- Jones PW. COPD 2005; 2:75–79.
- Chupp GL et al. Poster presented at AAAI 2017; Poster L17.
- Global Strategy for Asthma Management and Prevention, Global Initiative for Asthma (GINA) 2017. Available from: www.ginasthma.org.
- Schwenkglenks M et al. Value Health 2003; 6:75–83.
- Ortega HG et al. N Engl J Med 2014; 371:1198–1207.
- Lugogo N et al. Clin Ther 2016; 38:2058–2070.
Nucala is a registered trademark of the GlaxoSmithKline Group of Companies





