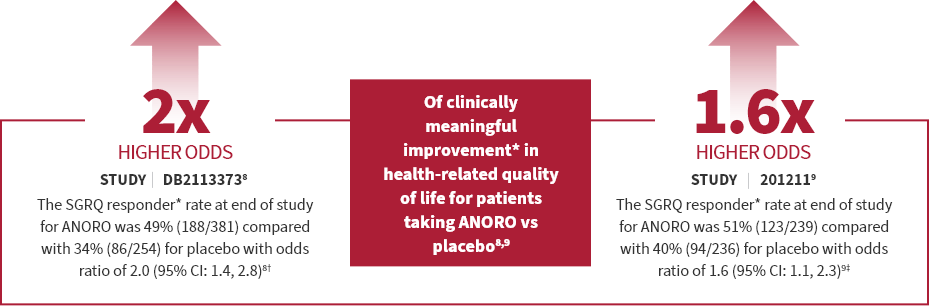
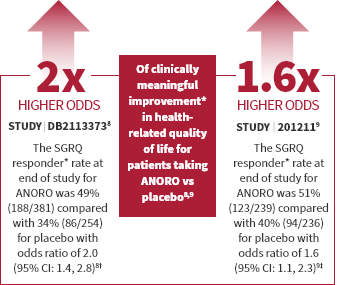
ANORO is for the maintenance treatment of patients with chronic obstructive pulmonary disease (COPD). ANORO is NOT for the relief of acute bronchospasm or for asthma.
ANORO is for the maintenance treatment of patients with chronic obstructive pulmonary disease (COPD).
ANORO is NOT for the relief of acute bronchospasm or for asthma.
ANORO is for the maintenance treatment of patients with chronic obstructive pulmonary disease (COPD).
ANORO is NOT for the relief of acute bronchospasm or for asthma.
ANORO is contraindicated in:
- patients with severe hypersensitivity to milk proteins or who have demonstrated hypersensitivity to umeclidinium, vilanterol, or any of the excipients.
CONTRAINDICATIONS
ANORO is contraindicated in:
- patients with severe hypersensitivity to milk proteins or who have demonstrated hypersensitivity to umeclidinium, vilanterol, or any of the excipients.
- use of a long-acting beta2-adrenergic agonist (LABA), including vilanterol, one of the active ingredients in ANORO, without an inhaled corticosteroid (ICS), in patients with asthma. ANORO is not indicated for the treatment of asthma.
CONTRAINDICATIONS
ANORO is contraindicated in:
- patients with severe hypersensitivity to milk proteins or who have demonstrated hypersensitivity to umeclidinium, vilanterol, or any of the excipients.
- use of a long-acting beta2-adrenergic agonist (LABA), including vilanterol, one of the active ingredients in ANORO, without an inhaled corticosteroid (ICS), in patients with asthma. ANORO is not indicated for the treatment of asthma.
WARNINGS AND PRECAUTIONS
- The safety and effectiveness of ANORO in patients with asthma have not been established. ANORO is not indicated for the treatment of asthma. Use of LABA as monotherapy (without ICS) for asthma is associated with an increased risk of asthma-related death, and in pediatric and adolescent patients, available data also suggest an increased risk of asthma-related hospitalization. These findings are considered a class effect of LABA monotherapy. Available data do not suggest an increased risk of death with use of LABA in patients with COPD.
- ANORO should not be initiated in patients during rapidly deteriorating or potentially life-threatening episodes of COPD.
- ANORO is NOT a rescue medication and should NOT be used for the relief of acute bronchospasm or symptoms. Acute symptoms should be treated with an inhaled, short-acting beta2-agonist.
- ANORO should not be used more often or at higher doses than recommended or with another LABA (eg, salmeterol, formoterol fumarate, arformoterol tartrate, indacaterol) for any reason, as an overdose may result. Clinically significant cardiovascular effects and fatalities have been reported in association with excessive use of inhaled sympathomimetic drugs, like LABA.
- Caution should be exercised when considering the coadministration of ANORO with ketoconazole and other known strong CYP3A4 inhibitors (including, but not limited to ritonavir, clarithromycin, conivaptan, indinavir, itraconazole, lopinavir, nefazodone, nelfinavir, saquinavir, telithromycin, troleandomycin, voriconazole) because increased cardiovascular adverse effects may occur.
- If paradoxical bronchospasm occurs, discontinue ANORO and institute alternative therapy.
- Hypersensitivity reactions such as anaphylaxis, angioedema, rash, and urticaria may occur after administration of ANORO. Discontinue ANORO if such reactions occur.
- Vilanterol can produce clinically significant cardiovascular effects in some patients as measured by increases in pulse rate, systolic or diastolic blood pressure, and also cardiac arrhythmias, such as supraventricular tachycardia and extrasystoles. If such effects occur, ANORO may need to be discontinued. ANORO should be used with caution in patients with cardiovascular disorders, especially coronary insufficiency, cardiac arrhythmias, and hypertension.
- Use with caution in patients with convulsive disorders, thyrotoxicosis, diabetes mellitus, and ketoacidosis, and in patients who are unusually responsive to sympathomimetic amines.
- Use with caution in patients with narrow-angle glaucoma. Instruct patients to contact a healthcare provider immediately if signs or symptoms of acute narrow-angle glaucoma develop.
- Use with caution in patients with urinary retention, especially in patients with prostatic hyperplasia or bladder-neck obstruction. Instruct patients to contact a healthcare provider immediately if signs or symptoms of urinary retention develop.
- Be alert to hypokalemia and hyperglycemia.
ADVERSE REACTIONS
- The most common adverse reactions (≥1% and more common than placebo) reported in four 6‑month clinical trials with ANORO (and placebo) were: pharyngitis, 2% (<1%); sinusitis, 1% (<1%); lower respiratory tract infection, 1% (<1%); constipation, 1% (<1%); diarrhea, 2% (1%); pain in extremity, 2% (1%); muscle spasms, 1% (<1%); neck pain, 1% (<1%); and chest pain, 1% (<1%).
- In addition to the 6-month efficacy trials with ANORO, a 12-month trial evaluated the safety of umeclidinium/vilanterol 125 mcg/25 mcg in subjects with COPD. Adverse reactions (incidence ≥1% and more common than placebo) in subjects receiving umeclidinium/vilanterol 125 mcg/25 mcg were: headache, back pain, sinusitis, cough, urinary tract infection, arthralgia, nausea, vertigo, abdominal pain, pleuritic pain, viral respiratory tract infection, toothache, and diabetes mellitus.
DRUG INTERACTIONS
- Caution should be exercised when considering the coadministration of ANORO with ketoconazole and other known strong CYP3A4 inhibitors as increased systemic exposure to vilanterol and cardiovascular adverse effects may occur. See prior Warning and Precaution regarding CYP3A4 inhibitors.
- ANORO should be administered with extreme caution to patients being treated with monoamine oxidase inhibitors, tricyclic antidepressants, or drugs known to prolong the QTc interval, or within 2 weeks of discontinuation of such agents, because they may potentiate the effect of vilanterol on the cardiovascular system.
- Use beta‐blockers with caution as they not only block the pulmonary effect of beta‐agonists, such as vilanterol, but may produce severe bronchospasm in patients with COPD.
- Use with caution in patients taking non–potassium-sparing diuretics, as ECG changes and/or hypokalemia associated with these diuretics may worsen with concomitant beta-agonists.
- Avoid coadministration of ANORO with other anticholinergic-containing drugs as this may lead to an increase in anticholinergic adverse effects.
Please see full Prescribing Information, including Patient Information, for ANORO.