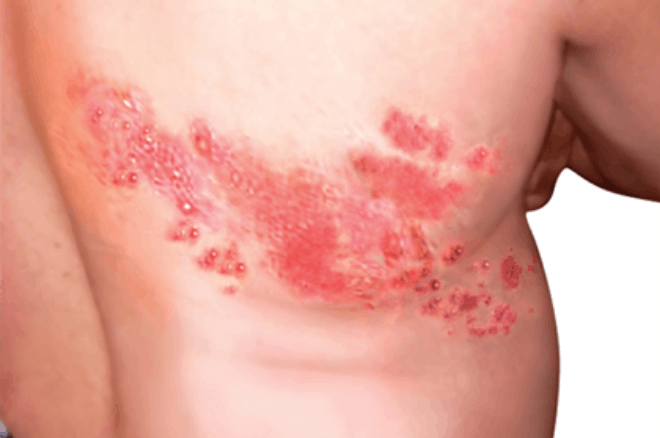
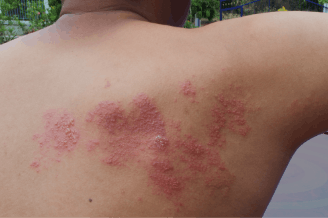
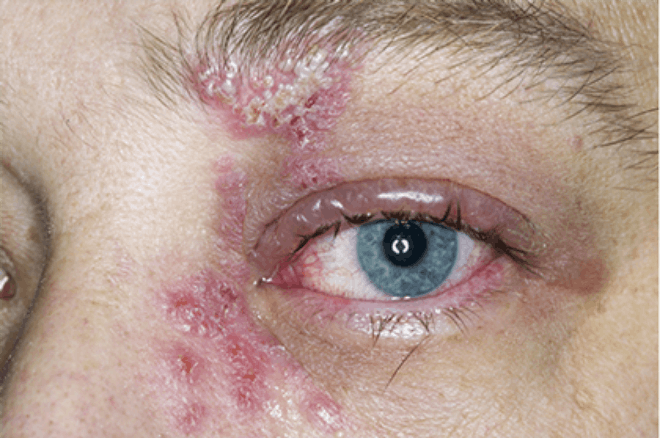
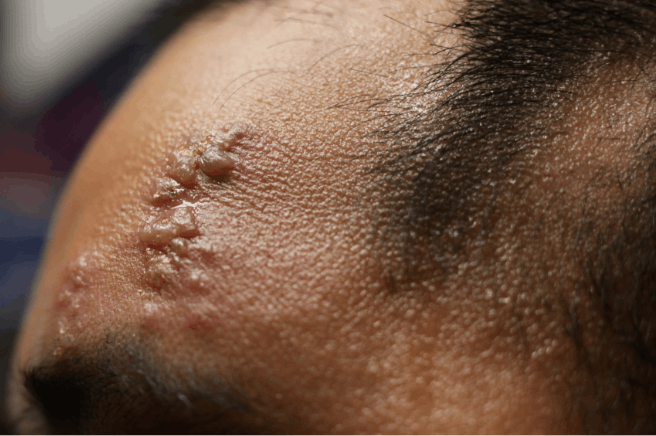
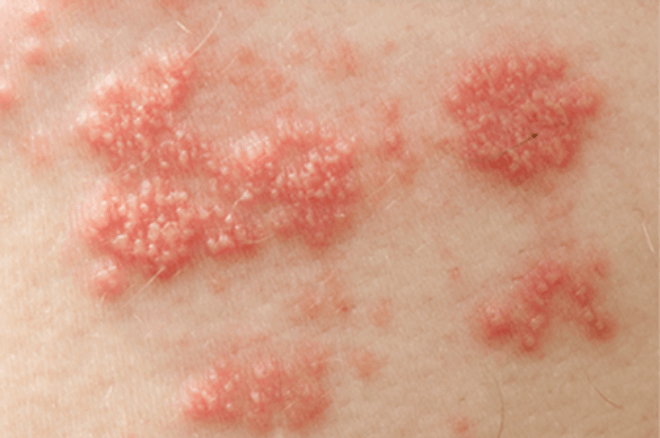
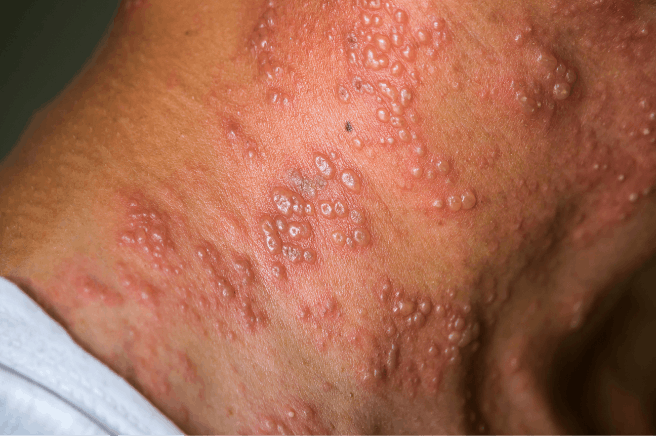
SHINGRIX is a vaccine indicated for prevention of herpes zoster (HZ) (shingles):
- in adults aged 50 years and older.
- in adults aged 18 years and older who are or will be at increased risk of HZ due to immunodeficiency or immunosuppression caused by known disease or therapy.
SHINGRIX is not indicated for prevention of primary varicella infection (chickenpox).
SHINGRIX is a vaccine indicated for prevention of herpes zoster (HZ) (shingles):
- in adults aged 50 years and older.
- in adults aged 18 years and older who are or will be at increased risk of HZ due to immunodeficiency or immunosuppression caused by known disease or therapy.
SHINGRIX is not indicated for prevention of primary varicella infection (chickenpox).
SHINGRIX is a vaccine indicated for prevention of herpes zoster (HZ) (shingles):
- in adults aged 50 years and older.
- in adults aged 18 years and older who are or will be at increased risk of HZ due to immunodeficiency or immunosuppression caused by known disease or therapy.
SHINGRIX is not indicated for prevention of primary varicella infection (chickenpox).
- SHINGRIX is contraindicated in anyone with a history of a severe allergic reaction (eg, anaphylaxis) to any component of the vaccine or after a previous dose of SHINGRIX
- Review immunization history for possible vaccine sensitivity and previous vaccination-related adverse reactions. Appropriate medical treatment and supervision must be available to manage possible anaphylactic reactions following administration of SHINGRIX
- SHINGRIX is contraindicated in anyone with a history of a severe allergic reaction (eg, anaphylaxis) to any component of the vaccine or after a previous dose of SHINGRIX
- Review immunization history for possible vaccine sensitivity and previous vaccination-related adverse reactions. Appropriate medical treatment and supervision must be available to manage possible anaphylactic reactions following administration of SHINGRIX
- SHINGRIX is contraindicated in anyone with a history of a severe allergic reaction (eg, anaphylaxis) to any component of the vaccine or after a previous dose of SHINGRIX
- Review immunization history for possible vaccine sensitivity and previous vaccination-related adverse reactions. Appropriate medical treatment and supervision must be available to manage possible anaphylactic reactions following administration of SHINGRIX
- In a postmarketing observational study, an increased risk of Guillain-Barré syndrome was observed during the 42 days following vaccination with SHINGRIX
- Syncope (fainting) can be associated with the administration of injectable vaccines, including SHINGRIX. Procedures should be in place to avoid falling injury and to restore cerebral perfusion following syncope
- Solicited local adverse reactions reported in individuals aged 50 years and older were pain (78%), redness (38%), and swelling (26%)
- Solicited general adverse reactions reported in individuals aged 50 years and older were myalgia (45%), fatigue (45%), headache (38%), shivering (27%), fever (21%), and gastrointestinal symptoms (17%)
- Solicited local adverse reactions reported in autologous hematopoietic stem cell transplant recipients (aged 18 to 49 and ≥50 years of age) were pain (88% and 83%), redness (30% and 35%), and swelling (21% and 18%)
- Solicited general adverse reactions reported in autologous hematopoietic stem cell transplant recipients (aged 18 to 49 and ≥50 years of age) were fatigue (64% and 54%), myalgia (58% and 52%), headache (44% and 30%), gastrointestinal symptoms (21% and 28%), shivering (31% and 25%), and fever (28% and 18%)
- The data are insufficient to establish if there is vaccine-associated risk with SHINGRIX in pregnant women
- It is not known whether SHINGRIX is excreted in human milk. Data are not available to assess the effects of SHINGRIX on the breastfed infant or on milk production/excretion
- Vaccination with SHINGRIX may not result in protection of all vaccine recipients