This is a fictional patient for illustrative purposes.
Anoro Ellipta is indicated as a once-daily maintenance bronchodilator treatment to relieve symptoms in adult patients with COPD.2
Explore Anoro Ellipta’s data vs. tiotropium/olodaterol1
In an 8-week open-label crossover study in patients not on maintenance therapy (for ≥2 weeks prior to randomisation), Anoro Ellipta demonstrated a 1.4x superior lung function improvement vs. tiotropium/olodaterol1 (with an extra 52mL improvement in trough FEV1)1
Trough FEV1 at week 8 in adults with moderate COPD* (ITT population)1
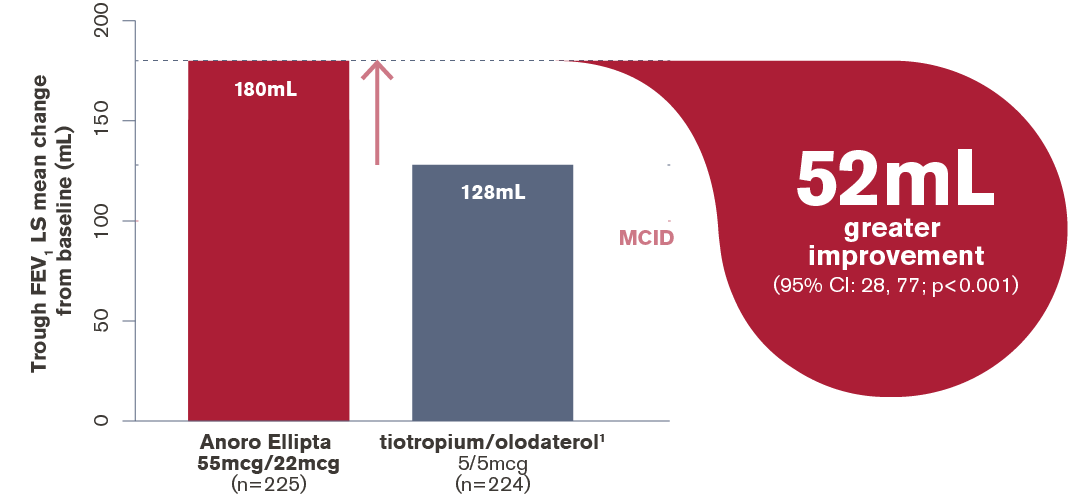

*Post bronchodilator FEV1 ≤70% to ≥50% predicted, mMRC ≥2;1
ITT, intention to treat; LS, least squares; MCID (minimum clinically important difference) = 100mL
Adapted from Feldman et al. 20171
ITT, intention to treat; LS, least squares;
Adapted from Feldman et al. 20171
Get started with Anoro Ellipta
References
- Feldman G.J et al. Adv Ther 2017; 34:2518–2533
- ANORO Ellipta SPC https://www.medicines.ie/medicines/anoro-ellipta-55-micrograms-22-micrograms-inhalation-powder-pre-dispensed-31288/spc Accessed: July 2024
Anoro Ellipta is generally well tolerated. Consistent with its antimuscarinic activity, Anoro should be used with caution in patients with urinary retention or with narrow-angle glaucoma.2
Cardiovascular effects, such as cardiac arrhythmias e.g. atrial fibrillation and tachycardia, may be seen after the administration of muscarinic receptor antagonists and sympathomimetics, including Anoro.2
Therefore, Anoro should be used with caution in patients with severe cardiovascular disease.2
Common adverse reactions include: headache, urinary tract infection, sinusitis, nasopharyngitis, pharyngitis, upper respiratory tract infection, cough, oropharyngeal pain, constipation, dry mouth.2
Adverse events should be reported directly to the Health Products Regulatory Authority (HPRA) on their website: www.hpra.ie.
Adverse events should also be reported to GlaxoSmithKline on 1800 244 255.
Trade marks are owned by or licensed to the GSKI group of companies.

PM-IE-UCV-WCNT-240005 July 2024








