Nucala reduced the rate of exacerbations* by 53% compared with placebo at 32 weeks in the MENSA study (Primary endpoint: 0.83 Nucala (n=194) vs. 1.74 placebo (n=191) (95% CI: 36–65) p<0.001).6
This page contains real world evidence. Greg is a real patient diagnosed with eosinophilic asthma at 24. He was prescribed Nucala in July 2017.
Nucala is indicated as an add-on treatment for severe refractory eosinophilic asthma in adults, adolescents and children aged 6 years and older.2
Nucala provides real-life protection from exacerbations and maintenance OCS vs. baseline whilst reducing eosinophils to normal levels1,3-5
Nucala reduced median daily OCS dose by 50% at 24 weeks in the SIRIUS study (Secondary endpoint: 50% Nucala (n=69) (95% CI: 20.0–75.0) vs. 0% placebo (n=66) (95% CI: -20.0–33.3) p=0.007).7
France ATU real-world study
France ATU was a retrospective observational study in 146 patients receiving mepolizumab for severe eosinophilic asthma across 20 centres in France


Baseline | 5.8 Exacerbations per year
At 2 years | 0.8 exacerbations per year with Nucala
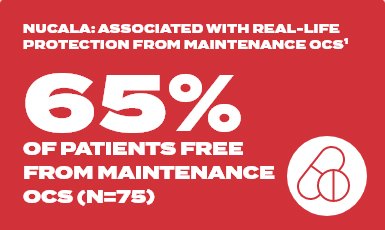

Baseline | 18 out of 146 patients free from OCS
At 2 years | 49 out of 75 patients free from OCS
Results are descriptive.
In the 24-week Phase III study, SIRIUS, the secondary outcome of total cessation in OCS use was not significant (N (%); Nucala 10/69 (14%) vs. 5/66 (8%) placebo p=0.41). However, the protocol did not allow for patients on higher doses (25mg/day or more) to be weaned completely.7
COSMEX long term study
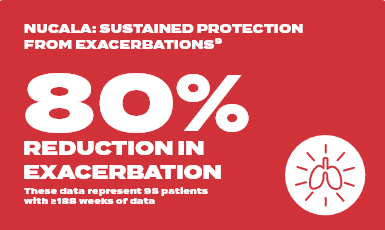

Pre-treatment | 5.02 exacerbations per year
Week 188 | 0.98 exacerbations per year with Nucala
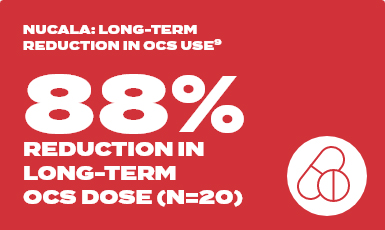

Baseline | 11.3 mg/day
Week 128 | with Nucala 1.3 mg/day
In total, 95 patients with ≥188 weeks of continuous reporting across MENSA, COSMOS and COSMEX with ≤12 weeks between the last dose in COSMOS and first dose in COSMEX are summarised (MENSA: placebo [n=24], Nucala [n=71]). The Nucala group in MENSA contains patients on both 100mg SC and 75mg IV doses (75mg IV dose is not an approved dose of Nucala). Analyses include clinically significant exacerbations from MENSA and all exacerbations from COSMOS and COSMEX.
What role do eosinophils play in health and disease?

Eosinophils are the driving force behind up to 79% of cases of severe asthma, however they also have potential multi-functional roles in health.12-18

When choosing a treatment for severe eosinophilic asthma, consider Nucala, the only anti-IL-5 which reduces eosinophils to normal levels.2-4

NUCALA PRE-FILLED PEN2
Nucala is the only once-monthly asthma biologic with a simple fixed dose.2
The recommended dose of Nucala is 100mg SC once every 4 weeks in adults and adolescents 12 years and older, available in a pre-filled pen, prefilled syringe or lyophilised powder. The licensed dose of Nucala in children aged 6–11 years is 40mgSC regardless of weight and available in lyophilised powder.
Identify and support patients with severe eosinophilic asthma

Identify patients with elevated eosinophil levels that are eligible for Nucala
Have a question?
Footnotes
*Defined as deterioration in asthma requiring use of systemic corticosteroids and/or an ED visit and/or hospital admission.
Real-world studies are designed to evaluate associations among variables and not to definitively establish causality. These limitations are important when interpreting results: lack of comparator arm, differences in patient populations and data collection vs. randomised controlled trials.²¹ France ATU (cohort of patients in France) was a retrospective observational study.1
Nucala is generally well tolerated. Very commonly or commonly reported adverse reactions in clinical trials included: headache; back pain; local injection site reactions; systemic administration-related and hypersensitivity reactions (which can occur after a long duration of treatment); LRTI; UTI; pharyngitis; nasal congestion; upper abdominal pain; eczema and pyrexia.
The long-term safety and immunogenicity profile of Nucala was similar to that observed in placebo-controlled asthma trials.8
References
- Taillé C et al. Eur Respir J 2020; in press (https://doi.org/10.1183/13993003.02345-2019) Last accessed: May 2022
- Nucala SmPC, from www.medicines.ie Last Accessed: May 2022
- Lugogo N et al. Clinical Therapeutics 2016; 38;2058–2070
- Yancey SW et al. J Allergy Clin Immunol 2017; 140:1509–1518
- Hartl S et al. Eur Respir J 2020; 1–34
- Ortega HG et al. N Engl J Med 2014; 371:1198–1207
- Bel EH et al. N Engl J Med 2014; 371:1189–1197
- Khurana S et al. Clin Ther 2019; 41:2041–2056
- Heredia JE, et al. Cell 2013;153;376–388
- Wu D, et al. Science 2011;332;243–7
- Zhu L, et al. PloS one 2013;8;e67613;1–6
- Yang J, Torio A, Donoff RB, et al. Depletion of eosinophil infiltration by anti-IL-5 monoclonal antibody (TRFK-5) accelerates open skin wound epithelial closure. Am J Pathol. 1997;151(3):813-819.
- Ramirez GA, et al. Biomed Res Int 2018:9095275
- Wenzel SE, et al. Am J Respir Crit Care Med 1999;160:1001–1008
- de Carvalho-Pinto RM, et al. Resp Med 2012;106:47–56
- Haldar P, et al. Am J Respir Crit Care Med 2008;178:218–224
- Albers FC, et al. J Asthma 2018;55:152–160
- Weller F & Spencer LA Nat Rev Immunol 2017;17:746–760
- Wen T & Rothenberg E. Microb Spectr 2016;4:doi:10.1128/microbiolspec.MCHD-0020-2015
- Leather DA et al. Adv Ther 2020; 1–21
Adverse events should be reported directly to the Health Products Regulatory Authority (HPRA) on their website: www.hpra.ie . Adverse events should also be reported to GlaxoSmithKline on 1800 244 255.
© 2023 GSK group of companies or its licensor.
Trade marks are owned by or licensed to the GSK group of companies.
PM-IE-MPL-WCNT-200012 | May 2023







