Nucala: The only asthma biologic with a fixed dose for either adults (100mg/month) or children from 6.y.o. (40mg/month)1
Nucala targets IL-5 and is indicated as an add-on treatment for adult, adolescent and paediatric patients (aged 6 or above) with severe refractory eosinophilic asthma.1

Dosing and Administration¹
| Patients aged ≥18 years | |
|---|---|
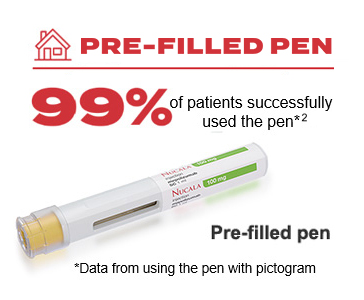
| Form | Pre-filled pen |
| Administration | 100 mg subcutaneously |
| Injection site | Self-administration: abdomen |
Nucala is indicated as an add-on treatment for severe refractory eosinophilic asthma in adults, adolescents and children aged 6 years and older. Only Nucala 100mg SC is licenced for use.1
Nucala is generally well tolerated. In clinical trials, Nucala had a similar incidence of adverse events vs. placebo with the exception of injection site reactions (8% vs. 3%), which occurred mainly within the first 3 injections1
The recommended dose of Nucala is 100mg SC once every 4 weeks in adults and adolescents 12 years and older, available in a pre-filled pen, pre-filled syringe or lyophilised powder. The licensed dose of Nucala in children aged 6-11 years is 40mg SC regardless of weight and available in lyophilised powder.1
References
- Nucala SmPC, Available from: www.medicines.ie Last Accessed: January 2024.
- Bernstein D et al. Usability of mepolizumab single-use prefilled autoinjector for patient self-administration. J Asthma 2019; 28:1–12. doi: 10.1080/02770903.2019.1630641 [Epub ahead of print].
Adverse events should be reported directly to the Health Products Regulatory Authority (HPRA) on their website: www.hpra.ie ;. Adverse events should also be reported to GlaxoSmithKline on 1800 244 255.
© 2024 GSK group of companies or its licensor.
Trade marks are owned by or licensed to the GSK group of companies.
PM-IE-MPL-WCNT-200017 | January 2024








