How Nucala works
Nucala is indicated as an add-on treatment for severe refractory eosinophilic asthma in adults, adolescents and children aged 6 years and older. Only Nucala 100mgs SC is licensed for use.1
Rethink IL5 Rethink Nucala (mepolizumab)
This video illustrates the mechanism of disease and the role of IL5 in eosinophils and beyond.
POM. Further information available from GlaxoSmithKline, 12 Riverwalk, Citywest, Business Campus, Dublin 24. Tel: 01-4955000
PM-IE-MPL-WCNT-240002 Date of prep February 2025.
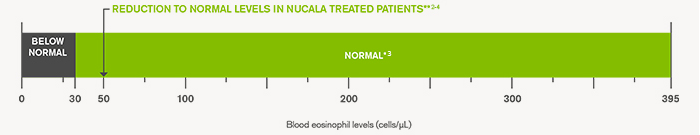
*The normal range is based on 5th – 95th percentile values from the general population3
**geometric mean value from COSMOS study. This is a 52 week, open label extension study which evaluated the long term safety and efficacy of mepolizumab. (n=590; SD logs 0.934)2
Nucala is indicated as an add-on treatment for severe refractory eosinophilic asthma in adults, adolescents and children aged 6 years and older. Only Nucala 100mg SC is licensed for use1
Nucala is generally well tolerated. Very commonly or commonly reported adverse reactions in clinical trials included: headache; back pain; local injection site reactions; systemic administration-related and hypersensitivity reactions (which can occur after a long duration of treatment); LRTI; UTI; pharyngitis; nasal congestion; upper abdominal pain; eczema and pyrexia.1
The recommended dose of Nucala is 100mg SC once every 4 weeks in adults and adolescents 12 years and older, available in a pre-filled pen, pre-filled syringe or lyophilised powder. The licensed dose of Nucala in children aged 6-11 years is 40mg SC regardless of weight and available in lyophilised powder.1
References
- Nucala SmPC, Available on www.medicines.ie Last accessed Jan 2025.
- Lugogo N et al. Long-term efficacy and safety of mepolizumab in patients with severe eosinophilic asthma: a multi-center, open-label, Phase IIIb study. Clin Ther 2016; 38:2058–2070.
- Hartl S et al. Blood eosinophil count in the general population: typical values and potential confounders. Eur Respir J 2020; pii: 1901874. doi: 10.1183/13993003.01874-2019 [Epub ahead of print].
- Yancey et al. 2017, J Allergy Clin Immunol, 140 (6), 1509-1518
- Khurana S et al. Long-term safety and clinical benefit of mepolizumab in patients with the most severe eosinophilic asthma: the COSMEX study. Clin Ther 2019; 41:2041–2056.
- Heredia JE;Cell;2013;153;376-388.
- Wu D;Science;2011;332;243-7.
- Zhu L;PloS one;2013;8;e67613;1-6.
- Yang Y, et al. Am J Pathol 1997;151:813-819.
- Ramirez GA, et al. Biomed Res Int. 2018;2018:9095275.
- Wenzel SE, et al. Am J Respir Crit Care Med. 1999;160:1001–1008.
- de Carvalho-Pinto RM, et al. Resp Med. 2012;106:47–56.
- Haldar P, et al. Am J Respir Crit Care Med. 2008;178:218–224.
- Albers FC, et al. J Asthma. 2018;55:152–160
- Wen T, Rothenberg ME. The regulatory function of eosinophils. Microbiol Spectr 2016; 4 doi: 10.1128/microbiolspec. MCHD-0020-2015.
- Weller PF, Spencer LA. Functions of tissue-resident eosinophils. Nat Rev Immunol 2017; 17:746–760.
Adverse events should be reported directly to the Health Products Regulatory Authority (HPRA) on their website: www.hpra.ie. Adverse events should also be reported to GlaxoSmithKline on 1800 244 255.
GSK group of companies or its licensor.
Trade marks are owned by or licensed to the GSK group of companies.
PM-IE-MPL-WCNT-250002 | March 2025









