Request a demo device
Help your patients try the Ellipta device by ordering a demo device
Not a healthcare professional? Visit our Public site
Not a healthcare professional? Visit our Public site

has been added to your basket
59
Trelegy Ellipta (fluticasone furoate/umeclidinium/vilanterol) is indicated as a maintenance treatment in adult patients with moderate to severe chronic obstructive pulmonary disease (COPD) who are not adequately treated by a combination of an inhaled corticosteroid (ICS) and a long acting β2-agonist (LABA) or a combination of a LABA and a long-acting muscarinic antagonist (LAMA).1
Patients with COPD who were naïve to both Ellipta and the comparator device made fewer critical errors using an Ellipta device compared with other common COPD inhalers after reading the PIL*2
*Defined as errors that are likely to result in no or significantly reduced or minimal medication being delivered to the lung after reading the patient information leaflet only
Open label, single visit, placebo inhaler, crossover comparison in patients naïve to Ellipta and the comparator inhalers (p<0.001 for all comparisons) n=567
In another trial, the INTREPID trial, the % of patients with ≥1 critical error in inhalation technique at week 24 was: 6% in the FF/UMEC/VI treatment group, 3% in the non-Ellipta multiple inhaler triple therapy group. OR: 1.99; p=0.103 (NS)3
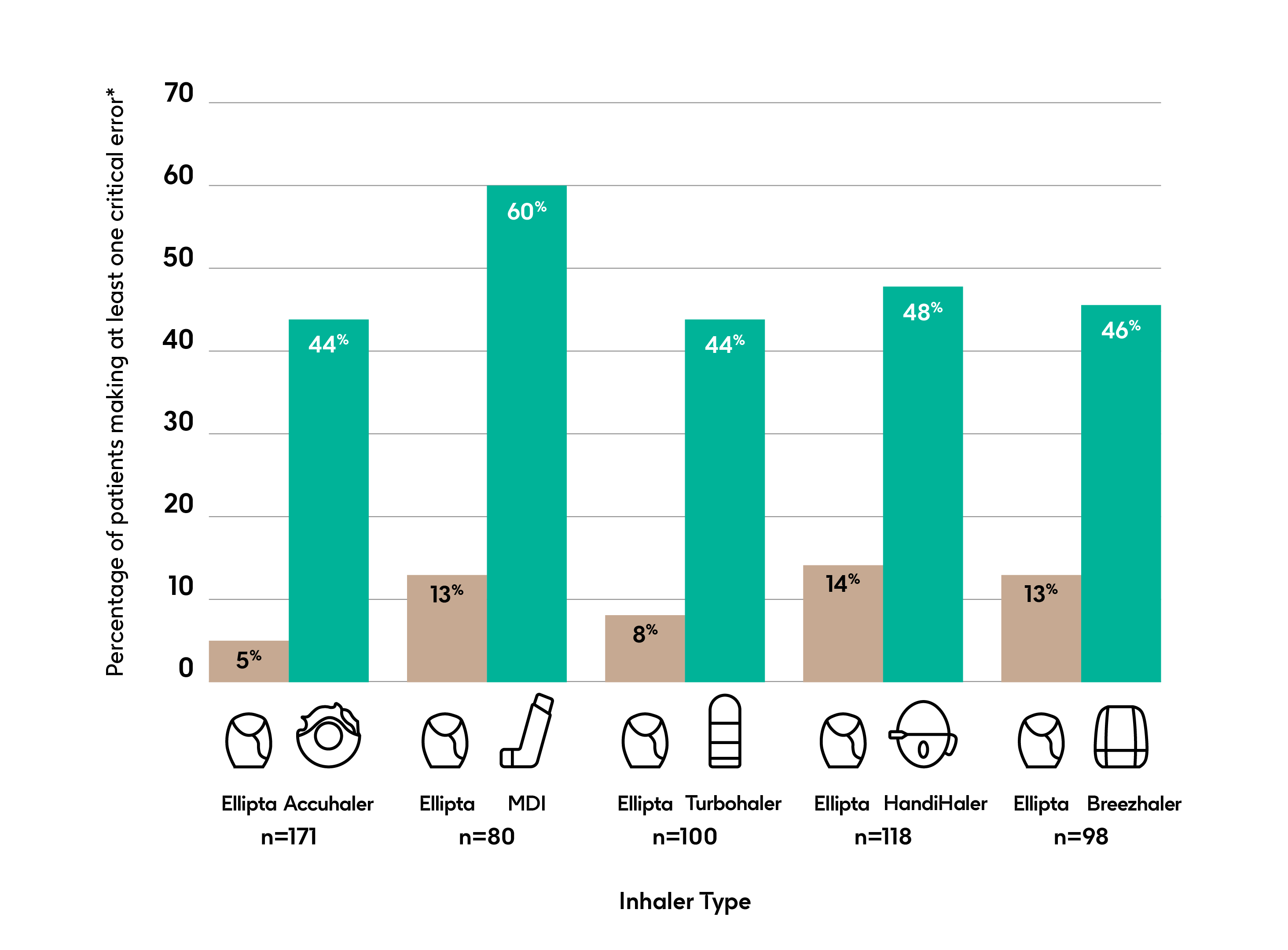
Adapted from Van der Palen J et al (2016).
Help your patients try the Ellipta device by ordering a demo device

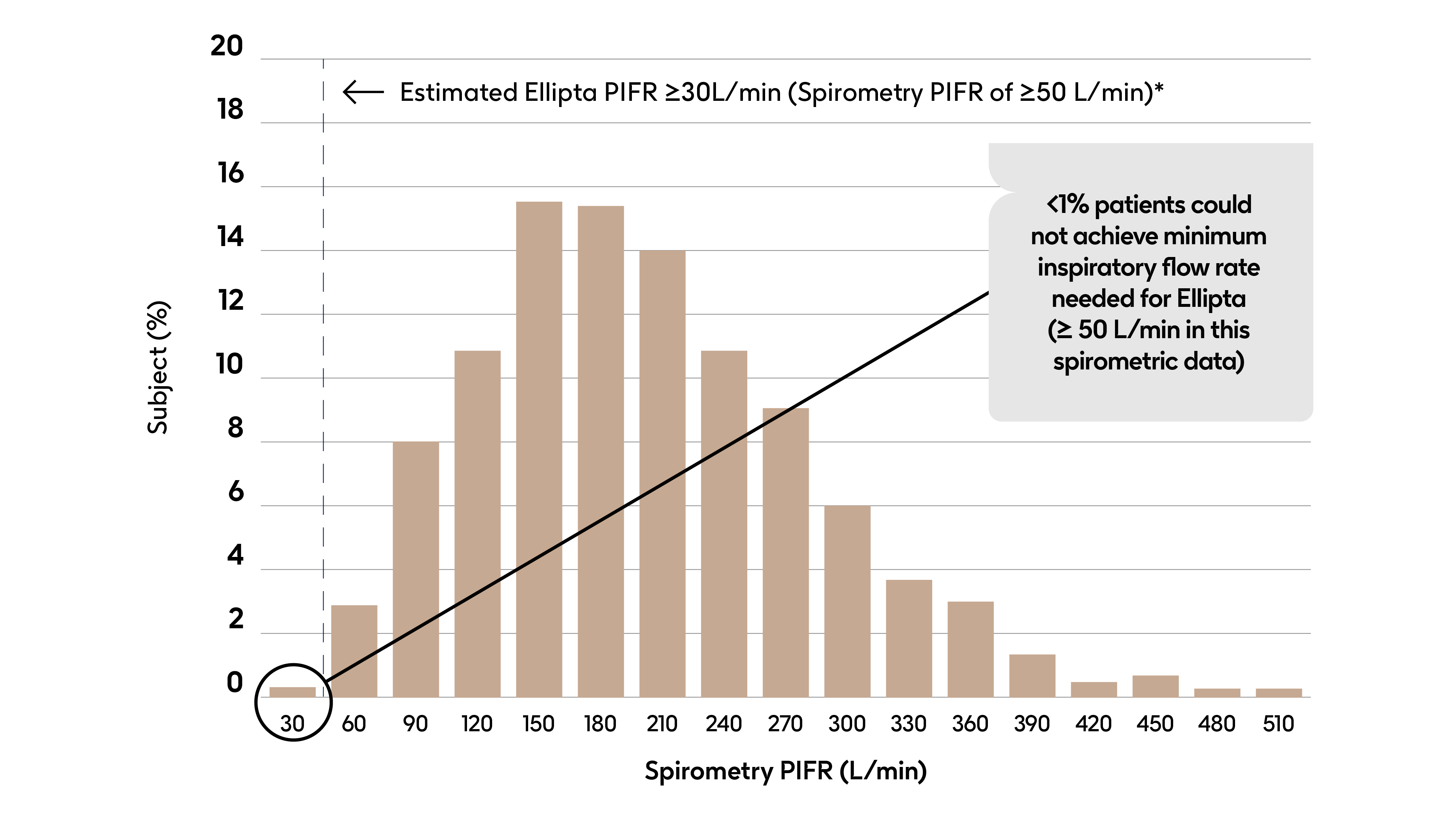
With Ellipta, >99% of patients can generate the inspiratory flow required to ensure that even severe COPD patients can get the dose they need4,5
Analysis of spirometry data for 1,951 patients with moderate/severe COPD
*Spirometry PIFR of 50 L/min correlated to a PIFR of 30 L/min for the Ellipta device, a flow rate which has been shown in-vitro to be adequate for appropriate dose delivery6
Treatment effect is not dependent on PIF
This training whistle helps patients understand the correct inspiratory flow needed to use the Ellipta device. It produces a whistle sound when the necessary inspiratory flow is achieved.

Patients learn to use an Ellipta faster than other common COPD inhalers.2
Open label, single visit, placebo inhaler, crossover comparison in patients naïve to Ellipta and the comparator inhalers. n=567
Time to teach based on reading the PIL followed by nurse instruction if required.
Number of instructions from trained respiratory nurse: After reading the PIL, the majority of patients (57-70%) made no errors using the Ellipta inhaler, thus not requiring instruction from the nurse.2
The majority of patients required nurse instruction for the other inhalers, for example 85% in the case of the MDI.2
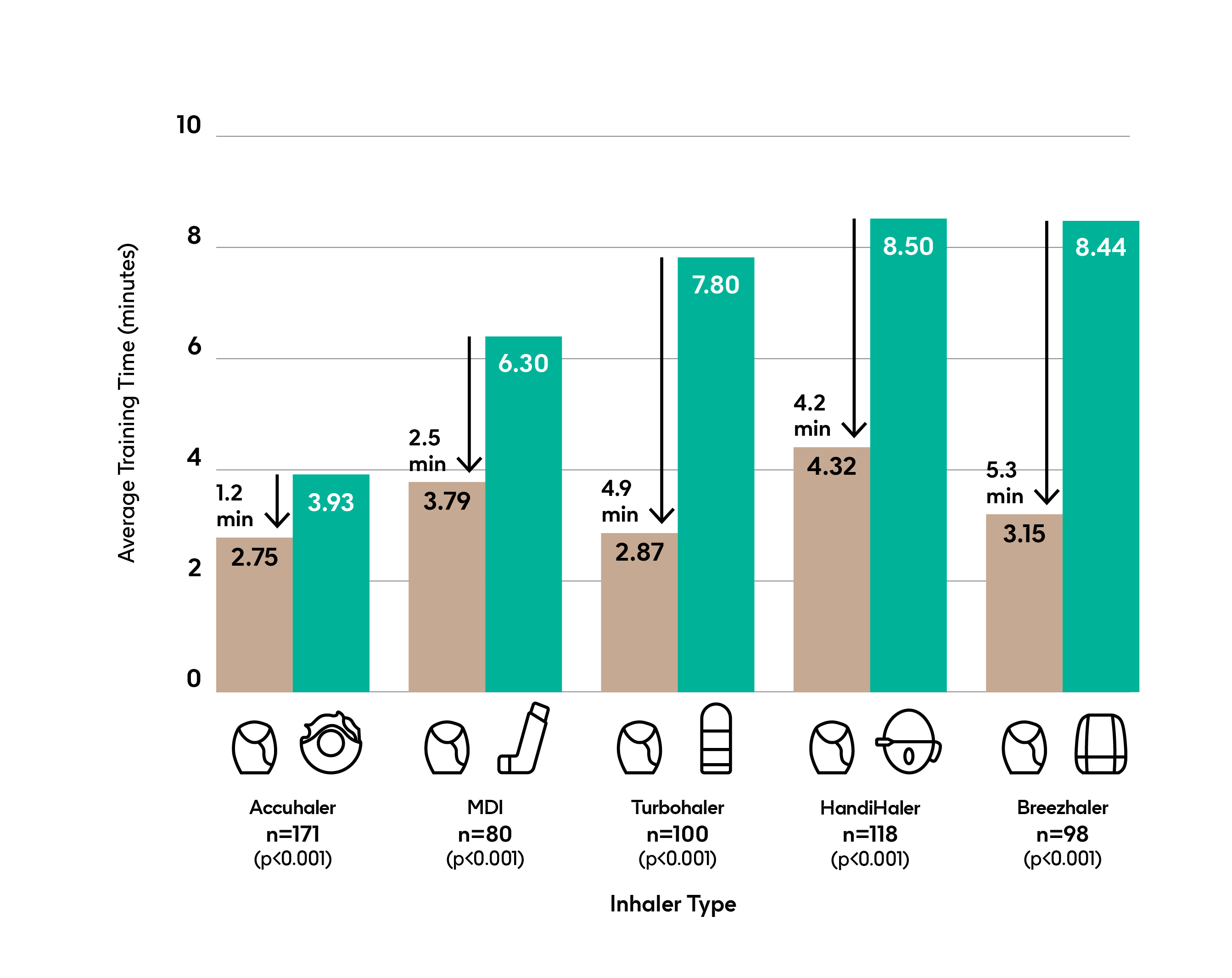
Adapted from Van der Palen J et al (2016).
This guide can support your eligible COPD patients who have been prescribed Trelegy Ellipta

Sustained bronchodilation over 24 hours 12-week, non-inferiority study involving 1017 patients. Data determined in a subcohort analysis (n=148).7
Demonstrated rapid & sustained bronchodilation over 24-hours8,9
Not licensed as a monotherapy in COPD.
Data from a Phase1 study (n=36) on healthy individuals8 and a Phase II study (n=602) on COPD patients9

Up to 24 hour anti-inflammatory activity in vitro10 Clinical significance of the data is not known.
FF is not licenced as a monotherapy in COPD.
Side effects: Common (≥1/100 to <1/10): pneumonia, upper respiratory tract infection, bronchitis, pharyngitis, rhinitis, sinusitis, influenza, nasopharyngitis, candidiasis of mouth and throat, urinary tract infection, headache, cough, oropharyngeal pain, constipation, arthralgia, back pain. Other important side effects include Uncommon (≥1/1,000 to <1/100): viral respiratory tract infection, dysgeusia, dysphonia, dry mouth, fractures, supraventricular tachyarrhythmia, tachycardia, atrial fibrillation, vision blurred, glaucoma, eye pain; Rare(≥1/10,000 to <1/1,000): anxiety, tremor, intraocular pressure increased, muscle spasms, dysuria, hypersensitivity reactions, including anaphylaxis, angioedema, urticaria and rash; hyperglycaemia; palpitations and urinary retention. See Summary of Product Characteristics for other adverse reactions.1
Abbreviations
COPD, chronic obstructive pulmonary disease; FF, fluticasone furoate; MDI, metered dose inhaler; MITT, multiple-inhaler triple therapy; NS, non-significant; OR, odds ratio; PIL, patient information leaflet; UMEC, umeclidinium; VI, vilanterol PIF, peak inspiratory flow; PIFR, peak inspiratory flow rate
References
Adverse events should be reported. Reporting forms and information can be found at https://yellowcard.mhra.gov.uk/ or search for MHRA Yellowcard in the Google Play or Apple App store. Adverse events should also be reported to GSK on 0800 221 441 or UKSafety@gsk.com.
March 2025 | PM-GB-FVU-WCNT-250001 (V1.0)