
Watch a video comparison of Avamys spray viscosity versus 2 competitor INS's devices
How to use Avamys device video

A step by step guide to preparation and use of the Avamys nasal spray device.
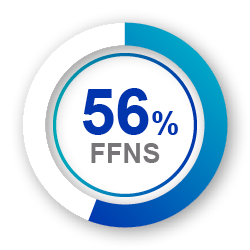
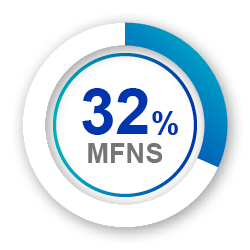
Avamys provides improved experience vs MFNS 5

Less drip down nose
or throat 5
(p<0.05 and p<0.001, respectively)

Less irritation 5
(p<0.001)

More soothing 5
(p<0.05)
Overall 5 patients (2%) reported AEs with FFNS and 13 patients (4%) with MFNS. Few common AEs were reported: rhinorrhoea, nasal discomfort, cough. No severe safety issues were identified during the study. 5
*Multicentre, randomised, double-blind, cross-over study where patient (n=300) preferences were determined by using three questionnaires
(Overall Preference, Immediate Attributes and Delayed Attributes).
AE: Adverse event; AR: Allergic rhinitis; FFNS: Fluticasone furoate nasal spray; FPNS: Fluticasone propionate nasal spray; MFNS: Mometasone furoate nasal spray.
References:
- Vasar M, Houle PA, Douglass JA, et al. Fluticasone furoate nasal spray: Effective monotherapy for symptoms of perennial allergic rhinitis in adults/adolescents. Allergy Asthma Proc. 2008;29(3);313-321.
- Avamys SmPC available on medicines.ie last accessed February 2025 https://www.medicines.ie/medicines/avamys-27-5-micrograms-spray-nasal-spray-suspension-31374/spc
- Meltzer EO, Stahlman JE, Leflein J, et al. Preferences of adult patients with allergic rhinitis for the sensory attributes of fluticasone furoate versus fluticasone propionate nasal sprays: A randomized, multicenter, double-blind, single-dose, crossover study. Clin Ther. 2008;30(2):271-279.
- Yonezaki M, Akiyama K, Karaki M, et al. Preference evaluation and perceived sensory comparison of fluticasone furoate and mometasone furoate intranasal sprays in allergic rhinitis. Auris Nasus Larynx. 2016;43(3):292-297.
- Yanez A, Dimitroff A, Bremner P, et al. A patient preference study that evaluated fluticasone furoate and mometasone furoate nasal sprays for allergic rhinitis. Allergy Rhinol. 2016;7(4):e183–e192.
Adverse events should be reported directly to the Health Products Regulatory Authority (HPRA) on their website: www.hpra.ie . Adverse events should also be reported to GlaxoSmithKline on 1800 244 255.
Avamys is a registered trademark of the GlaxoSmithKline group of companies







