
ARIA 2016 1
First-line treatment option for symptomatic patients with or without previous treatment.

AAOHNS 2015 2
Recommended for patients with a clinical diagnosis of AR where symptoms affect QoL.

BSACI 2017 3
Mainstay of anti-inflammatory intervention in AR and first-line therapy for moderate-to-severe persistent symptoms.

AAAAI / ACAAI 2017 4
Strongly recommends first-line use in patients ≥12 years of age (over OAH and INCS combination and LTRA) in SAR.
BSACI Guidelines 2017 3
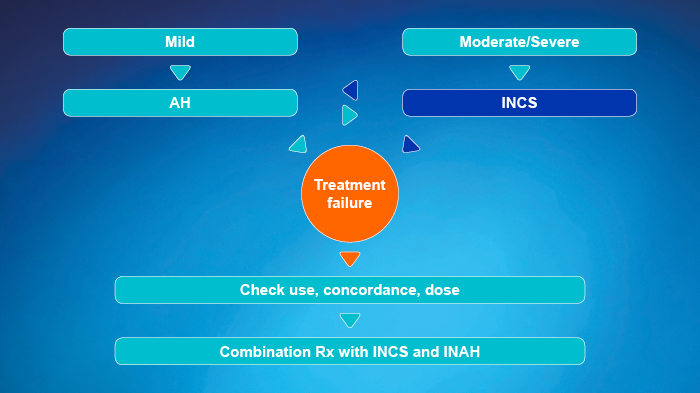
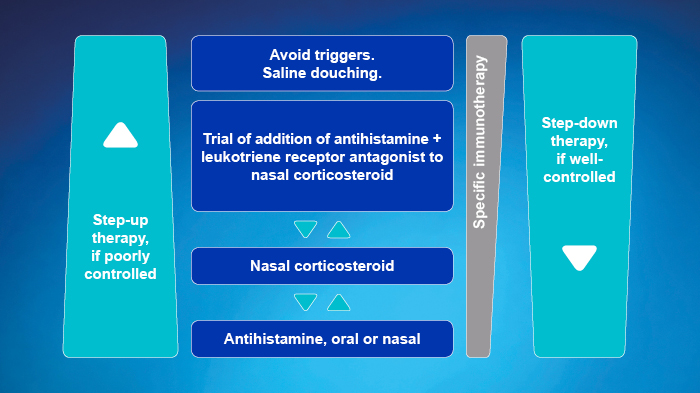
- Approach depends on the severity of rhinitis symptoms
- Use a step-up or step-down therapy, depending on control obtained in response to treatment
- OAH may be better tolerated, INAH have a more rapid onset of action
- Reconsider diagnosis if not controlled within 1 to 2 weeks
Footnotes:
AAAAI: American Academy of Allergy, Asthma and Immunology; AAOHNS: American Academy of Otolaryngology - Head and Neck Surgery; ACAAI: American College of Allergy, Asthma, and Immunology; AH: Antihistamine; AR: Allergic rhinitis; ARIA: Allergic Rhinitis and its Impact on Asthma; BSACI: British Society for Allergy and Clinical Immunology; INAH: Intranasal antihistamine; INCS: Intranasal corticosteroids; LTRA: Leukotriene receptor antagonist; OAH: Oral antihistamine; QoL: Quality of life; Rx: Medical prescription; SAR: Seasonal allergic rhinitis.
References:
- Brozek J, Bousquet J, Agache I, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines-2016 revision. J Allergy Clin Immunol. 2017;140(4):950-958.
- Seidman M, Gurgel RK, Lin SY, et al. Clinical Practice Guideline: Allergic Rhinitis. Otolaryngol Head Neck Surg. 2015:152(18) S1-S43.
- Scadding GK, Kariyawasam HH, Scadding G, et al. BSACI guideline for the diagnosis and management of allergic and non-allergic rhinitis. Clin Exp Allergy. 2017;47:856-889.
- Wallace DV, Dykewicz MS, Oppenheimer J, et al. Pharmacologic Treatment of Seasonal Allergic Rhinitis: Synopsis of Guidance from the 2017 Joint Task Force on Practice Parameters. Ann Intern Med. 2017;167(12):876-881.
- Santos A, Borrego LM, Rotiroti G, et al. The need for patient-focused therapy for children and teenagers with allergic rhinitis: a case-based review of current European practice. Clin Transl Allergy. 2015;5:2.
Adverse events should be reported directly to the Health Products Regulatory Authority (HPRA) on their website: www.hpra.ie. Adverse events should also be reported to GlaxoSmithKline on 1800 244 255.
Avamys is a registered trademark of the GlaxoSmithKline group of companies





