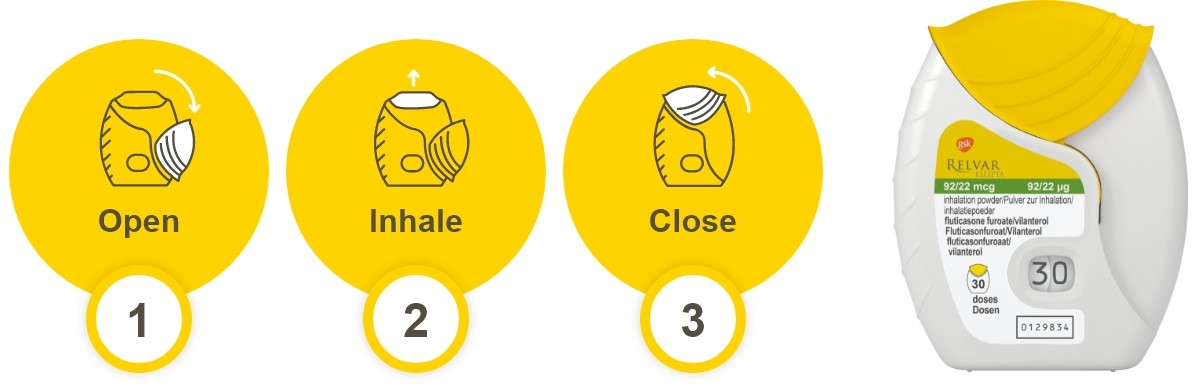

How does Relvar Ellipta compare with other devices?

Ellipta is preferred by patients vs Turbohaler, Diskus and MDI, with fewer patients making critical errors vs Turbohaler.2
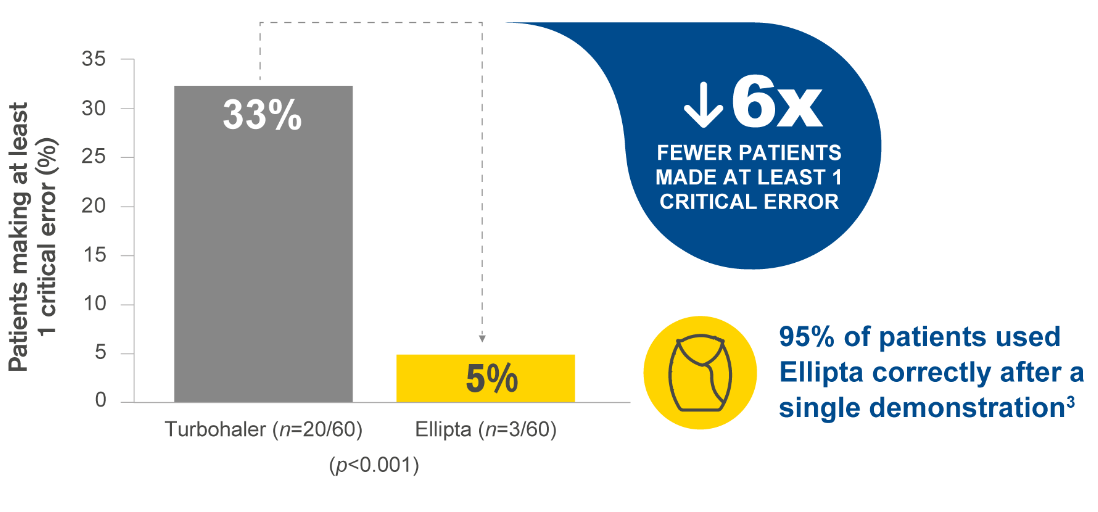

This graph has been independently created by GSK from the original data. The same results were first published in van der Palen J, et al. NJP Prim Care Respir Med 2016; 26:16079.
MDI and Diskus did not meet statistical significance.
Critical error defined as errors that are likely to result in no or minimal medication being inhaled.2
Discover Relvar
Need help with this product?
Abbreviations
MDI, metered dose inhaler.
- Global Datasheet Fluticasone furoate/vilanterol: v11, March 2020.
- van der Palen J, et al. NJP Prim Care Respir Med 2016;26:16079.
- Svedsater H, et al. NPJ Prim Care Respir Med 2014;24:14019.
RELVAR Ellipta was created in collaboration with

Adverse events should be reported directly to the Health Products Regulatory Authority (HPRA) on their website: www.hpra.ie . Adverse events should also be reported to GlaxoSmithKline on 1800 244 255.
Relvar is a registered trademark of the GlaxoSmithKline group of companies
PM-IE-FFV-WCNT-230009 June 2023











