Co-primary endpoints:
- Change from baseline in total endoscopic nasal polyps score at week 52.
- Change from baseline in mean nasal obstruction VAS score during Week 49-52.
Er du ikke helsepersonell? Besøk våre hjemmesider
Er du ikke helsepersonell?
Besøk gjerne våre sider for publikum.

Anoro Ellipta 20 mcg
har blitt lagt til din handelkurv.
59
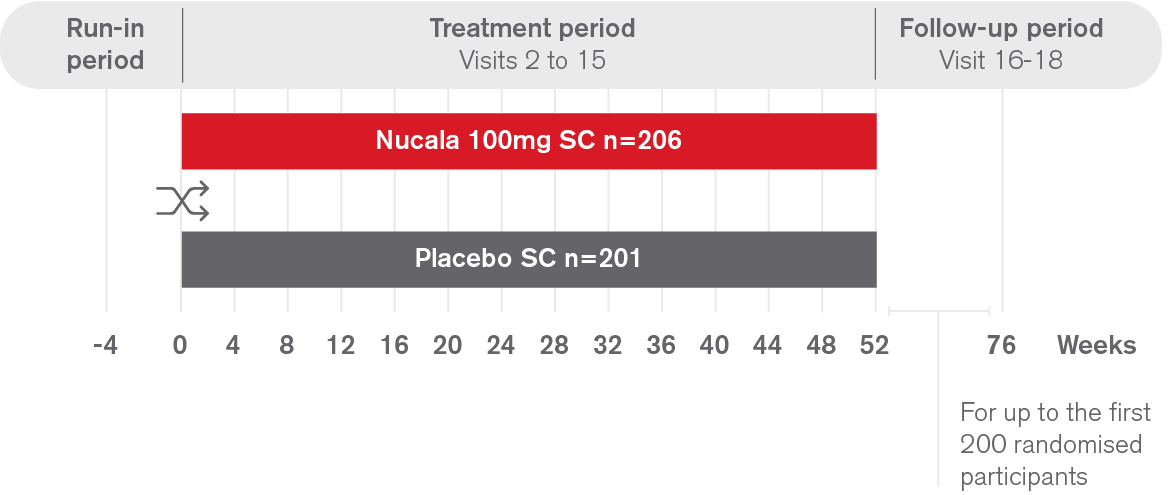
SYNAPSE is a 52-week, randomised, double-blind, parallel group Phase III study assessing the clinical efficacy and safety of Nucala 100mg SC as an add-on to maintenance treatment in adults with severe bilateral nasal polyps, compared to placebo.1
Co-primary endpoints:
Secondary endpoints:
* Combining scores for nasal obstruction, nasal discharge, throat mucus, and loss of smell.1
Study population, n=407
Adults (≥ 18 years old) with a history of at least one prior surgery for nasal polyps, and recurrent nasal polyps despite treatment with current standard of care, and in need of surgery.
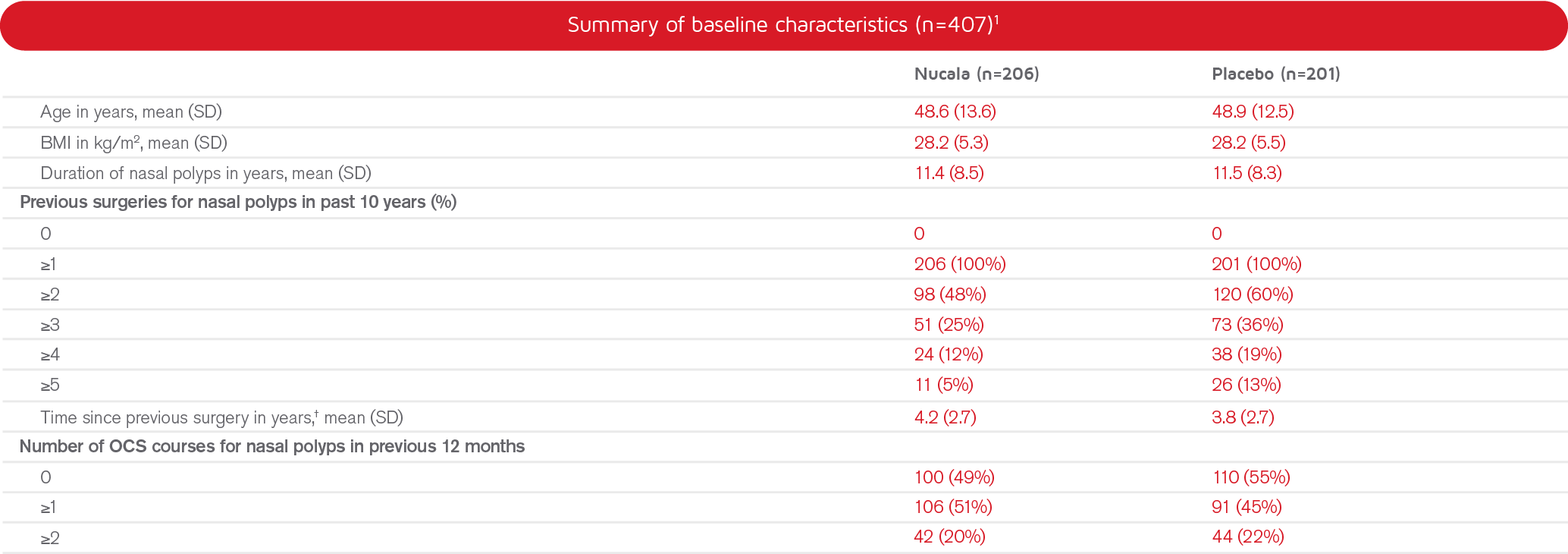
†Includes patients with partial dates for previous surgery; if day was missing, assumed as the last day of the month; if month was missing, assumed as December.1
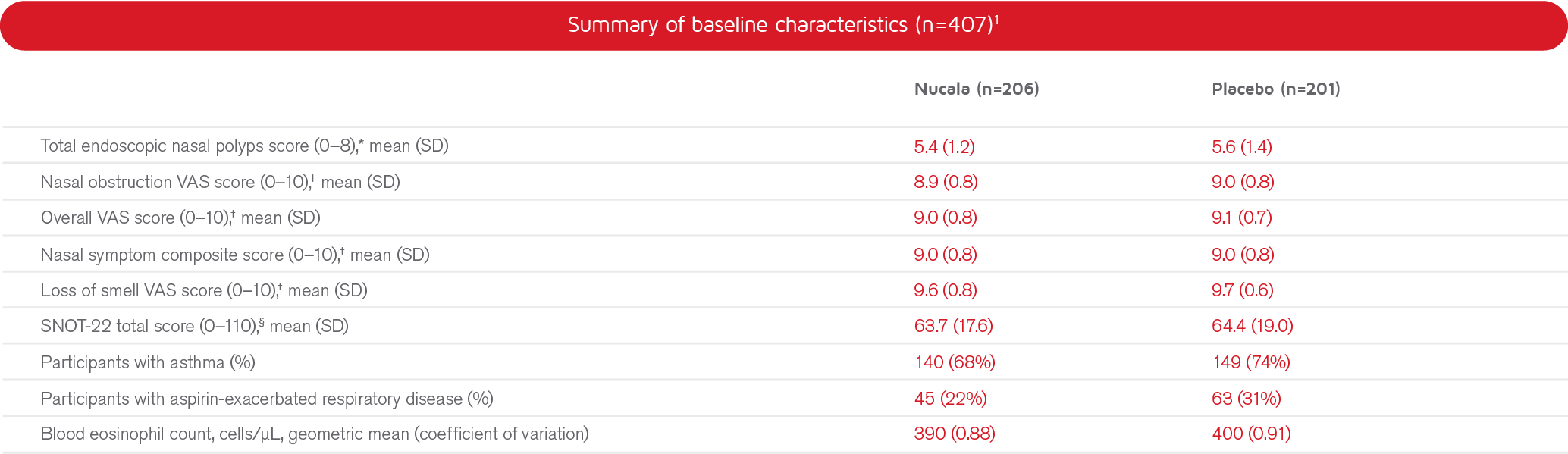
*Endoscopic NP score; total endoscopic NP score was the sum of the right and left nostril scores, ranging from 0 (no polyps) to 4 (large polyps causing complete obstruction of the interior meatus) for each nostril, giving a total score of up to 8.1
†A VAS was used to collect participant perceived symptom data. Participants were asked to indicate on a VAS the severity of 5 nasal polyps symptoms daily (one VAS for each symptom) and symptoms overall: please rate your “[symptom]” at its worst over the previous 24 hours. Symptoms were: 1) nasal obstruction; 2) nasal discharge; 3) feeling of mucus in the throat; 4) loss of smell; 5) facial pain; 6) nasal polyps symptoms. The VAS score could range from 0.0 to 10.0.1,2 Higher scores indicate greater disease severity or worse quality of life.1
‡Combining scores for nasal obstruction, nasal discharge, throat mucus and loss of smell. Higher scores indicate greater disease severity or worse quality of life.1
§SNOT-22 is a 22-item self reported questionnaire developed to measure symptoms and impacts related to chronic rhinosinusitis. Score range is from 0 to 110, with each item scored from 0 (no problem) to 5 (the problem is as bad as it can be). SNOT-22 items were summarized across 6 domains: nasal symptoms, non-nasal symptoms, ear/facial symptoms, sleep, fatigue, and emotional consequences.3 Higher scores indicate greater disease severity or worse quality of life.1
Warnings/Precautions: Should not be used to treat acute asthma exacerbations. Patients should be instructed to seek medical advice if their asthma remains uncontrolled or worsens after starting treatment. Abrupt discontinuation of corticosteroids after initiation of mepolizumab treatment is not recommended.
Allergic reactions: Acute and delayed systemic reactions, including hypersensitivity reactions, have occurred following administration of mepolizumab. Patients should be instructed to seek medical attention immediately if allergic reactions occur. In the event of a hypersensitivity reaction, appropriate treatment as clinically indicated should be initiated.
Parasitic infections: Pre-existing helminth infections should be treated before starting therapy. If patients become infected whilst receiving treatment with mepolizumab and do not respond to anti-helminth treatment, temporary discontinuation of therapy should be considered.
Organ-threatening or life-threatening manifestations of EGPA and HES: has not been studied.
Adverse reactions: In clinical studies in patients with severe refractory eosinophilic asthma and EGPA, headache, injection site reactions and back pain were the most commonly reported adverse reactions during treatment. In patients with CRSwNP: headache and back pain. In patients with HES: headache, urinary tract infection, injection site reactions and pyrexia.
Read the summary of product characteristics for more information before prescribing Nucala.
Adverse events should be reported to GlaxoSmithKline on phone: 22 70 20 00.
Video guidance prefilled Pen
Video guidance prefilled Syringe
BMI, body mass index; ENT, ear-nose-throat; NP, nasal polyps; OCS, oral corticosteroid; SC, subcutaneous; SD, standard deviation; SNOT-22, sino-nasal outcome test (22 items); VAS, visual analog score.
REFERENCES
PM-NO-MPL-WCNT-250005 December 2025