How to prescribe Tivicay?
TIVICAY (dolutegravir) is a once-daily, unboosted integrase inhibitor indicated in combination with other anti-retroviral medicinal products for the treatment of Human Immunodeficiency Virus (HIV) infected adults, adolescents and children above the age of ≥4 weeks and ≥3 kg. 1
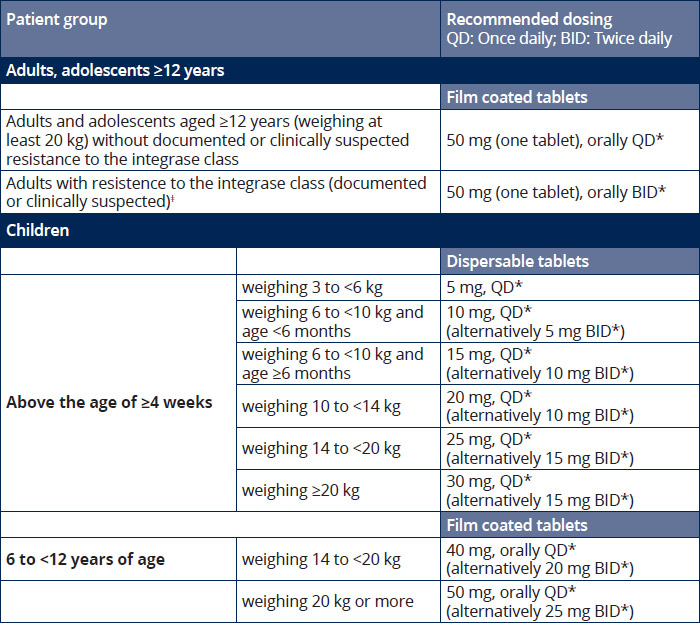
Dosing recommendations 1
Available tablet strengths 1
- 5 mg dispersible tablets: white, round, biconvex dispersible tablets approximately 6 mm in diameter
- 10 mg film-coated tablets: white, round, biconvex tablets approximately 6 mm in diameter
- 25 mg film-coated tablets: pale yellow, round, biconvex tablets approximately 7 mm in diameter
- 50 mg film-coated tablets: yellow, round, biconvex tablets approximately 9 mm in diameter
We emphasize that bioavailability of film-coated tablets and dispersible tablets is not comparable, and therefore can not be used interchangeably on a milligram-to-milligram basis. The dosage recommendations for the different formulations must be specifically followed.1
Tivicay should not be co-administered with polyvalent cation-containing antacids, supplements or multivitamins containing calcium, iron or magnesium. Tivicay is recommended to be administered 2 hours before or 6 hours after these medicinal products.
Dolutegravir increased metformin concentrations. A dose adjustment of metformin should be considered when starting and stopping coadministration of dolutegravir with metformin, to maintain glycaemic control. Metformin is eliminated renally and therefore it is of importance to monitor renal function when co-treated with dolutegravir.1
Contraindications 1
Hypersensitivity to the active substance or to any of the excipients. Co-administration with dofetilide.
Precaution
Women of childbearing potential should be counselled about the potential risk of neural tube defects with dolutegravir, including consideration of effective contraceptive measures. If a woman plans pregnancy, the benefits and the risks of continuing treatment with dolutegravir should be discussed with the patient.1
For further information, please see Tivicay preparatomtale
*Must be taken in combination with other antiretroviral agents. 1
**In the presence of INI class resistance, TIVICAY should preferably be taken with food to enhance exposure (particularly in patients with Q148 mutations). 1
‡In the presence of integrase inhibitor resistance, there are insufficient data to recommend a dose for dolutegravir in adolescents and children. 1
TIVICAY trademark is owned by or licensed to the ViiV Healthcare group of companies.







