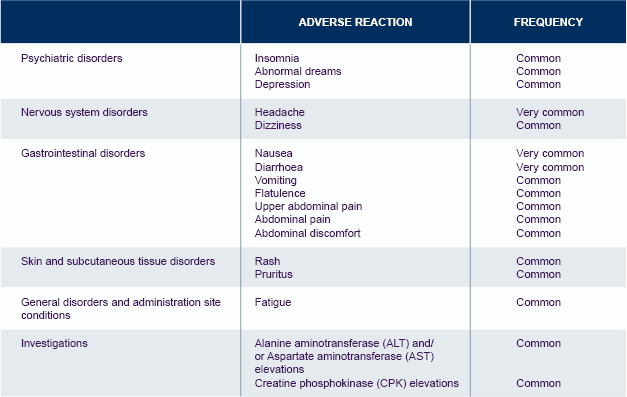
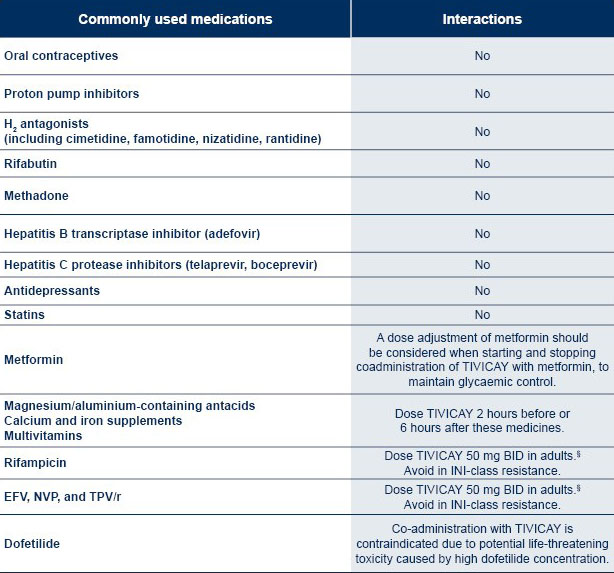
*The table is not exhaustive.
§In paediatric patients the weight-based once daily dose should be administered twice daily if used in combination with these medications.1
EFV: efavirenz
NVP: nevirapine
TPV/r: tipranavir/ritonavir
For a more comprehensive list of interactions, please see the HIV DDI database of University of Liverpool.
Contraindications
TIVICAY and dofetilide coadministration is contraindicated due to potential life-threatening toxicity caused by high dofetilide concentration.1
For further information on Tivicay safety, please see the Summary of Product Characteristics.
References:
- TIVICAY preparatomtale.
- Fantauzzi A, Turriziani O, Mezzaroma I. Potential benefit of dolutegravir once daily: efficacy and safety. HIV/AIDS (Auckl). 2013;5:29-40. doi: 10.2147/HIV.S27765
- Teixeira R, Nascimento Y, Crespo D. Safety aspects of protease inhibitors for chronic hepatitis C: adverse events and drug-to-drug interactions. Braz J Infect Dis. 2013;17(2):194-204.
- Patel P, Song I, Borland J, et al. Pharmacokinetics of the HIV integrase inhibitor S/GSK1349572 co-administered with acid- reducing agents and multivitamins in healthy volunteers. J Antimicrob Chemother. 2011;66(7):1567-1572.
- Kandel CE, Walmsley S. Dolutegravir - a review of the pharmacology, efficacy, and safety in the treatment of HIV. Drug Design, Development and Therapy. 2015:9 3547-3555. doi: 10.2147/DDDT.S84850
TIVICAY trademark is owned by or licensed to the ViiV Healthcare group of companies.






