
LEAVE NO EC PATIENT BEHIND
For patients with EC, the future of treatment may be redefined by immunotherapy. Knowing their MMR/MSI status will help make sure they are ready.
~1 in 3 patients with EC have tumours that are dMMR/MSI-H1-3
Testing for dMMR/MSI-H is crucial
EC has the highest rate of dMMR/MSI-H in any cancer. Knowing your patients’ status can help determine eligibility for immunotherapy.1-4
Recurrent/advanced EC is associated with poor survival5-8
Survival rates for patients with recurrent/advanced EC are low and decline dramatically with recurrence.2,7

1 IN 8
PATIENTS WITH EC AT ANY STAGE WILL HAVE A RECURRENCE5,7,9

17%
5-YEAR SURVIVAL RATE FOR RECURRENT/ADVANCED PATIENTS*2
*United States–based data.
Patients with EC need effective second-line options
Ninety percent of uterine cancers—the most common type of gynaecological cancer—are endometrial, making up more than 130,000 new cases in Europe in 2020.10,11 Currently, there is no established standard of care for post-platinum patients, and ORR drops dramatically between first- and second-line treatment.12,13
ORR <20% ON SINGLE-AGENT CHEMOTHERAPY†13,14
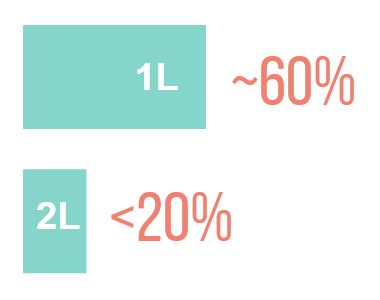
†ORR up to 27% has been reported in patients treated with paclitaxel who have been previously exposed to the treatment.15
Getting patients with EC ready for the future
Although therapeutic advancements have reached other tumour types,16-18 women with recurrent/advanced EC have had no standard of care in 2L treatment post-platinum therapy. But biomarker-driven therapeutic approaches could redefine the treatment paradigm.12
UNDERSTANDING THE ROLE OF MMR/MSI HAS THE POTENTIAL TO OFFER NEW TREATMENT OPTIONS FOR WOMEN WITH RECURRENT/ADVANCED EC4,19,20
Abbreviations
dMMR=mismatch repair deficient; EC=endometrial cancer; MMR=mismatch repair; MSI=microsatellite instability; MSI-H=microsatellite instability-high; ORR=overall response rate.
References
- Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357(6349):409-413.
- Makker V, Green AK, Wenham RM, Mutch D, Davidson B, Miller DS. New therapies for advanced, recurrent, and metastatic endometrial cancers. Gynecol Oncol Res Pract. 2017;4(19):1-12.
- The Cancer Genome Atlas Research Network. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497(7447):67-73.
- Luchini C, Bibeau F, Ligtenberg MJL, et al. ESMO recommendations on microsatellite instability testing for immunotherapy in cancer, and its relationship with PD-1/PD-L1 expression and tumour mutational burden: a systematic review-based approach. Ann Oncol. 2019;30(8):1232-1243.
- Fung-Kee-Fung M, Dodge J, Elit L, Lukka H, Chambers A, Oliver T; on behalf of the Cancer Care Ontario Program in Evidence-based Care Gynecology Cancer Disease Site Group. Follow-up after primary therapy for endometrial cancer: a systemic review. Gynecol Oncol. 2006;101(3):520-529.
- Morice P, Leary A, Creutzberg C, Abu-Rustum N, Darai E. Endometrial cancer. Lancet. 2016;387(10023):1094-1108.
- Huijgens ANJ, Mertens HJMM. Factors predicting recurrent endometrial cancer. Facts Views Vis Obgyn. 2013;5(3):179-186.
- Ueda SM, Kapp DS, Cheung MK, et al. Trends in demographic and clinical characteristics in women diagnosed with corpus cancer and their potential impact on the increasing number of deaths. Am J Obstet Gynecol. 2008;198(2):218.e1-e6.
- Tejerizo-Garcia A, Álvarez-Conejo C, Muñoz-Hernando L, et al. Tumor recurrence and tumor-related mortality in endometrial cancer: analysis in 276 patients. Indian J Cancer. 2015;52(4):682-684.
- World Health Organization International Agency for Research on Cancer (IARC). GLOBOCAN 2020: Cancer incidence and mortality statistics worldwide and by region. Accessed May 5, 2021. https://gco.iarc.fr/today/data/factsheets/cancers/24-Corpus-uteri-fact-sheet.pdf
- Felix AS, Brinton LA. Cancer progress and priorities: uterine cancer. Cancer Epidemiol Biomarkers Prev. 2018;27(9):985-994.
- Colombo N, Creutzberg C, Amant F, et al. & the ESMO-ESGO-ESTRO Endometrial Consensus Conference Working Group. ESMO-ESGO-ESTRO Consensus Conference on Endometrial Cancer: diagnosis, treatment and follow-up. Ann Oncol. 2016;27(1):16-41.
- Colombo N, Preti E, Landoni F, et al. on behalf of the ESMO Guidelines Working Group. Endometrial cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(suppl 6):vi33-vi38.
- Brooks RA, Fleming GF, Lastra RR, et al. Current recommendations and recent progress in endometrial cancer. CA Cancer J Clin. 2019;69(4):258-279.
- Lincoln S, Blessing JA, Lee RB, Rocereto TF. Activity of paclitaxel as second-line chemotherapy in endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2003;88:277-280.
- Vergote I, Denys H, De Greve J, et al. Treatment algorithm in patients with ovarian cancer. Facts Views Vis Obgyn. 2020;12(3):227-239.
- Mahmood RD, Morgan RD, Edmondson RJ, Clamp AR, Jayson GC. First-line management of advanced high-grade serous ovarian cancer. Curr Oncol Rep. 2020;22(64).
- Jazieh K, Bell R, Agarwal N, Abraham J. Novel targeted therapies for metastatic breast cancer. Ann Transl Med. 2020;8(14):907.
- Yen T-T, Wang T-L, Fader AN, Shih I-M, Gaillard S. Molecular classification and emerging targeted therapy in endometrial cancer. Int J Gynecol Pathol. 2019;39(1):26-35.
- Neri M, Peiretti M, Melis GB, et al. Systemic therapy for the treatment of endometrial cancer. Expert Opin Pharmacother. 2019;20(16):2019-2032.
Senast uppdaterad: Juni 2022. NP-SE-DST-WCNT-220004




